INTRODUCTION
The liver is a major vital organ, and liver cirrhosis has become a crucial global public health concern and one of the major causes of morbidity and mortality. In Korea, according to national data, the mortality rate in relation to liver disease in 2018 was 13.4/100,000 population, and liver disease was the 7th leading cause of death. However, this rate was lower than that of 2007, which was 15.0/100,000 population.
1 In addition, the mortality rate with cirrhosis had been remarkably decreased over time, from 16.8/100,000 population in 2000 to 4.5/100,000 population in 2018.
2
The main etiologies of cirrhosis are hepatitis B virus (HBV), hepatitis C virus (HCV), alcoholism, and nonalcoholic steatohepatitis (NASH). In 2015, 257 million people worldwide were diagnosed with chronic HBV, with an overall prevalence greater than 8%,
3 and the most common causes of cirrhosis have been related to HBV until recently.
4 In addition, after clustered outbreaks of HCV infection associated with reuse of disposable syringes in Korea, the increasing diagnosis rate of HCV is an emerging concern for public health.
56 Alcohol is another important risk factor for cirrhosis. From 2005 to 2015, the global age standardized prevalence of cirrhosis due to alcohol increased by 16.1%, compared with those of HBV (11.9%), HCV (14.2%), and others (9.9%).
7 Finally, cirrhosis is one of the risk factors of hepatocellular carcinoma (HCC) and increases development of the disease. More than half of all patients diagnosed with liver cancer have cirrhosis. Hence, because the occurrence of cirrhosis increases the risk of complications and mortality, the presence or absence of cirrhosis in patients with chronic liver disease is an important factor in predicting prognosis.
It is likely that higher prevalence and mortality rate of cirrhosis induce a socioeconomic burden. However, through introduction of vaccines and antiviral agents for hepatitis, the causes of cirrhosis seem to be changing over time. For instance, HCV infection and NASH are the recent causes responsible for the growing burden of cirrhosis. To date, many studies from other countries have reported changes and characteristics of liver cirrhosis,
8910 but no long-term, community-based study data were available in Korea. Hence, this study underpins the implication of liver cirrhosis in Korea. We described the epidemiology, complications associated with cirrhosis, and development of HCC in Daegu and Gyeongbuk provinces with various causes. Through this study, we underline several important and changing epidemiological aspects and characteristics of patients with liver cirrhosis in Korea.
METHODS
Data source and study population
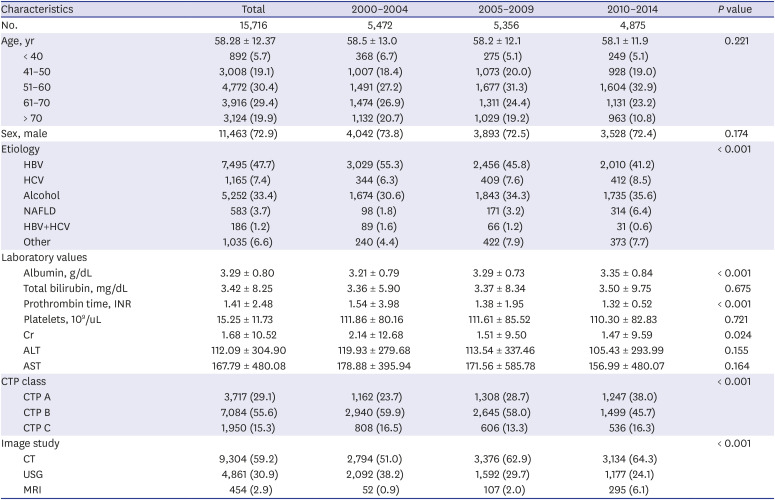
We retrospectively reviewed the medical records of all patients first diagnosed with cirrhosis in 5 university hospitals in Daegu-Gyeongbuk province during the 15 years from January 2000 to December 2014 (n = 15,716) and retrospectively followed these patients until March 2016. The five university hospitals were Keimyung University, Yeungnam University, Kyungpook University, Catholic University of Daegu, and Dongguk University. The data were collected from the medical records of patients with Korean Standard Classification of Diseases (KCD)-6 codes related to liver cirrhosis, recorded even once in any inpatient or outpatient encounter for the first time and were evaluated in each group at annual intervals. The KCD-6 codes used for diagnosis were K70.30 (alcoholic cirrhosis NOS), K70.31 (alcoholic cirrhosis with ascites), K70.2 (alcoholic fibrosis and sclerosis of liver), K71.7 (toxic liver disease with fibrosis and cirrhosis of liver), K74.0 (hepatic fibrosis), K74.2 (hepatic fibrosis with hepatic sclerosis), and K74.6 (other and unspecified cirrhosis of the liver). We only included patients newly diagnosed with KCD-6 code during the study period.
To confirm the incidence of HCC in cirrhosis patients, the diagnosis of HCC was based on any record of KCD-6 code. Diagnosis of HCC during the follow-up period was set as the primary end point. The incidence of HCC excluded those with diagnosis of HCC within 6 months after initial diagnosis of cirrhosis considering the screening period of 6 months. Because patients with HCC diagnosed within 6 months almost had HCC at the time of cirrhosis diagnosis. Patients who did not develop HCC were censored at the date of last follow up or death.
Definition of cirrhosis and etiologies of cirrhosis
The diagnosis of cirrhosis was made on clinical biochemical, histological, and radiological evidence accompanied by thrombocytopenia and clinical signs of cirrhosis complications such as esophageal varices, ascites, and hepatic encephalopathy. Although cirrhosis is a clinically and pathologically defined disease, all patients in this study were diagnosed with cirrhosis according to KCD code.
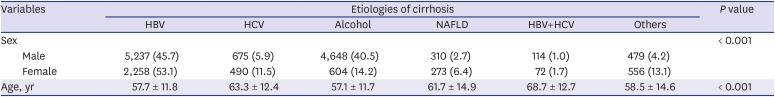
Among patients with liver cirrhosis, we categorized four etiologies. First, HBV cirrhosis was defined as cirrhosis with presence of hepatitis B surface antigen (HBsAg). Alcoholic cirrhosis was defined as cirrhosis in a patient who drinks excessively, defined as an average alcohol consumption exceeding 40 g/day in male and 20 g/day in female, and no other viral hepatitis.
11 HCV cirrhosis was defined as cirrhosis with anti-HCV antibody. Non-alcoholic fatty liver disease (NAFLD) was diagnosed by KCD code (K75.8, nonalcoholic steatohepatitis) in those who did not have HBV, HCV, or alcoholic liver disease defined as above. Finally, all patients not meeting the above criteria were classified as others; for example, those with autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cirrhosis.
These definitions were mutually exclusive by study design to compare the causes of cirrhosis.
Subgroup analysis for HCC incidence among cirrhosis patients
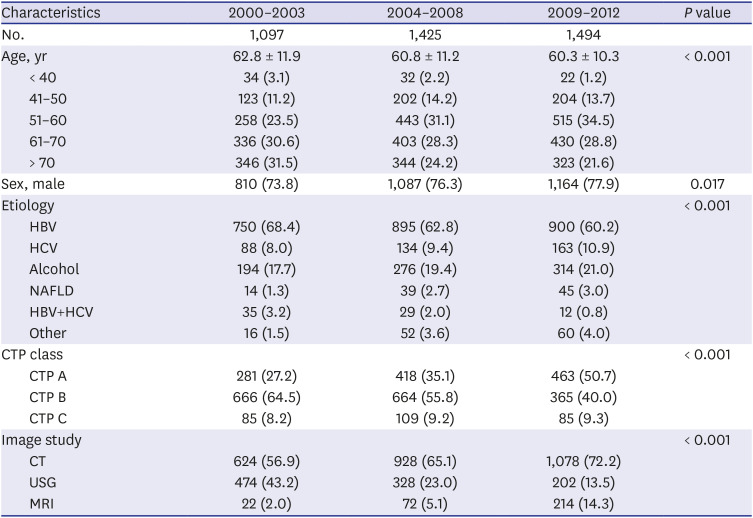
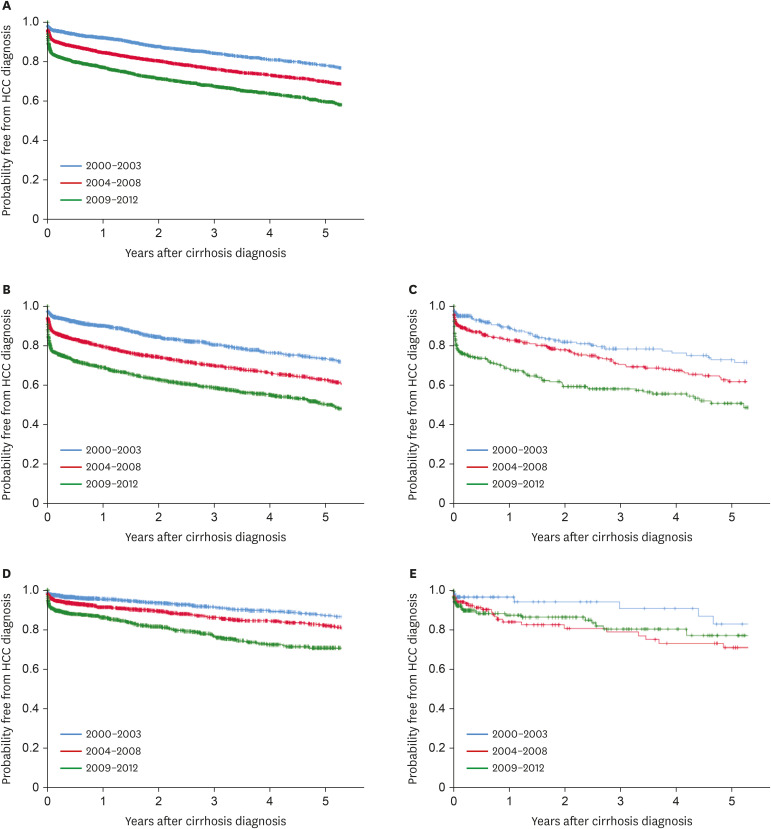
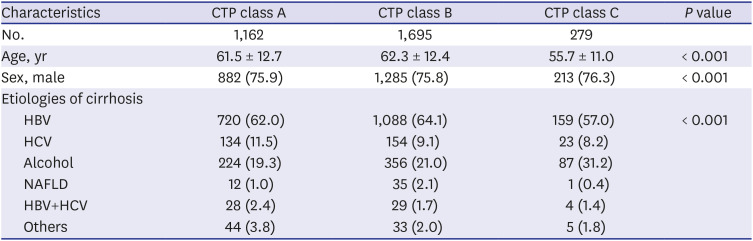
For evaluating HCC incidence, patients were divided into three groups according to time of diagnosis: 2000–2003, 2004–2008, and 2009–2012. We extracted age, sex, etiologies of cirrhosis, Child-Turcotte-Pugh (CTP) class, and performed imaging studies, then compared each periods.
Statistical analysis
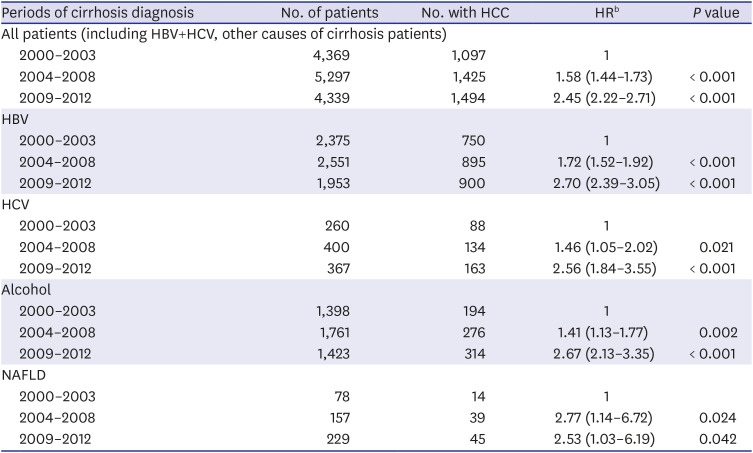
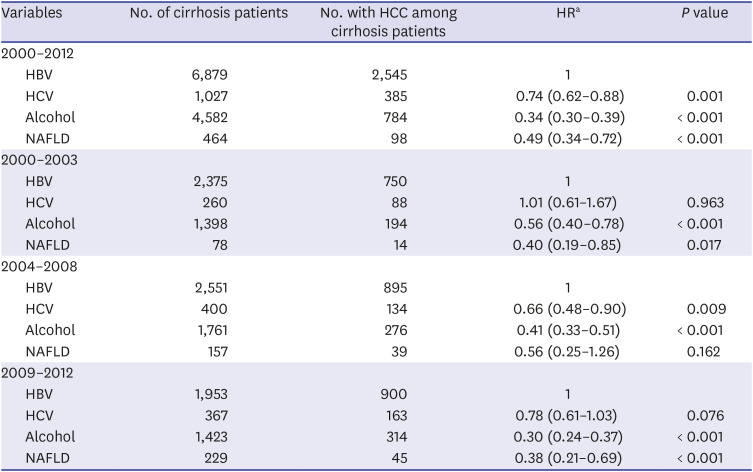
All statistical analyses were performed using SPSS v. 21.0 (IBM Corp., Chicago, IL, USA). A χ2 test was used for categorical variables and Fisher's exact test when appropriate. Continuous variables with skewed distribution were analyzed using the Mann-Whitney test. P < 0.05 was considered statistically significant. We used multivariate Cox proportional hazards regression to estimate the risk of HCC in patients diagnosed with cirrhosis according to period (2000–2003, 204–2008, and 2009–2012) and based on etiology in a limited maximum follow-up of 5 years. In addition, we used multivariable Cox proportional hazards regression to compare patients with HBV to those with HCV, alcohol, or NAFLD regarding the risk of HCC after cirrhosis diagnosis.
Ethics statement
This retrospective, multi-center clinical study was approved by Keimyung University's Institutional Review Board (IRB File No. 2014-08-002). Informed consent was waived by IRB.
DISCUSSION
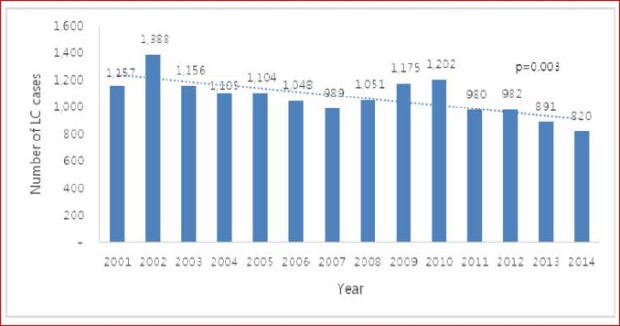
Despite an increasing total number of patients who visited an outpatient clinic in a tertiary hospital from national data,
12 the number of patients diagnosed with cirrhosis significantly decreased from 2001 to 2014 in this study. This is likely the result of some significant interventions: a high rate of domestic hepatitis B vaccination, a recent improvement in the awareness of hepatitis and antiviral drugs.
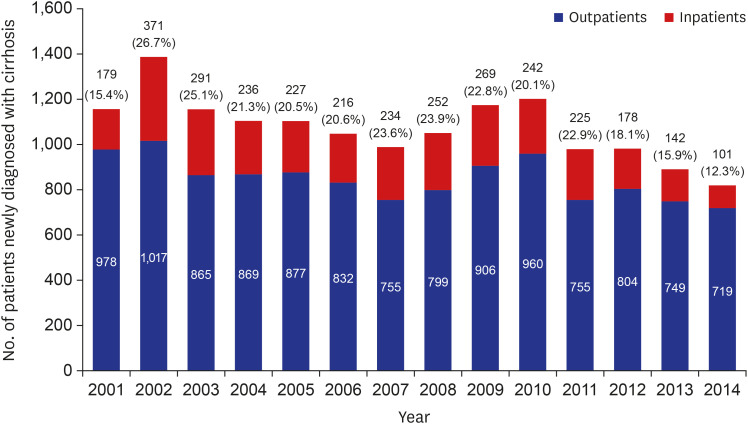
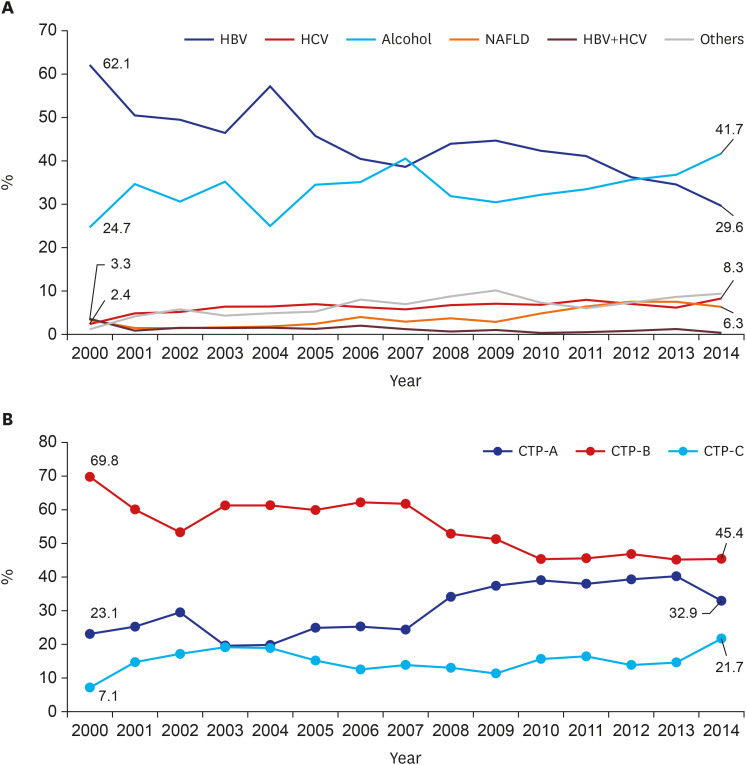
In this study, we analyzed a total of 15,716 cirrhosis patients with change in etiologies. Cirrhosis patients decreased in age over the 15 study years. This is because hospital accessibility has improved, and high-quality accurate imaging allows easy and fast cirrhosis diagnosis. Hence, many younger patients with chronic liver disease visited a hospital for regular follow up, and healthy young people undergo routine medical checkups, increasing early diagnosis of cirrhosis. Among these cirrhosis patients, the most common cause of cirrhosis in this study was HBV. In Korea, the estimated prevalence of HBV infection was about 3% in the 2016 Korea National Health and Nutrition Examination Survey (KNHANES).
13 Since dramatic changes have been made in the treatment of chronic HBV infection after introduction of the HBV vaccination, nationwide screening, and advances in antiviral treatment, the incidence of HBV cirrhosis has decreased over recent years. According to our expectation, development of cirrhosis and deaths due to cirrhosis associated with hepatitis B will continue to decline.
On the other hand, alcoholic cirrhosis was found to be increasing in this study. Alcohol consumption has increased in recent years in Korea. According to WHO reports, alcohol consumption per capita (15+) in Korea has risen from 24th in 2010 to 21st in 2016 among 34 OECD countries. Age-standardized death rates of cirrhosis due to alcohol in 2016 were 18.5 males and 4.5 females per 100,000 population. These values are relatively higher than those of neighboring countries, of which Japan's were 10.9/4.3, and China's were 14.6/8.3.
14 The social culture of Korea may contribute to this because drinking is considered a social activity and a part of everyday business. In addition, drinking scenes and advertisements for alcohol beverages are commonly portrayed through broadcasting systems. To date, there have been reports about a significant increase in social costs due to excessive alcohol consumption, which is an important cause of cirrhosis in Korea.
1516 We should increase social awareness by establishing effective frameworks for monitoring and surveillance of excessive alcohol consumption. If not, alcohol-related disease, especially cirrhosis, will continue to remain a problem and contribute to increasing economic burden.
Similar to alcoholic cirrhosis, HCV cirrhosis significantly increased over the study period. In Korea, hepatitis C was classified as a Sentinel Surveillance System infectious disease before 2017, though it was difficult to estimate the exact disease burden. During that time, the prevalence of HCV infection in Korea was reported to be 1%–2%.
1718 However, the prevalence of patients diagnosed with KCD-6 codes in health care facilities was less than 0.2% compared to the prevalence of diagnoses based on antibodies.
6 Before 2017, only about 25%–30% of all hepatitis C infections were diagnosed due to low recognition of the disease. To date, there is low awareness of hepatitis C in not only the general public, but also among doctors. In addition, because most HCV-infected patients have no symptoms, they are diagnosed late and already have complications such as cirrhosis or HCC. Hence, HCV was responsible for approximately 10%–15% of patients with cirrhosis and liver cancer in Korea. In this study, HCV was responsible for 8.3% of patients with cirrhosis in 2014, and the incidence is steadily increasing. Fortunately, hepatitis C has emerged as a public issue that requires a national response based on several outbreaks of HCV infection due to reuse of disposable syringes in health care facilities. After HCV infection was included in the Mandatory Surveillance System of infectious disease, diagnosis has been rapidly increasing. Therefore, strict quality control of invasive procedures in medical institutions and non-medical institutions is carried out at the national level to lower the prevalence of HCV infection and HCV cirrhosis. Moreover, if an economic evaluation of the hepatitis C screening strategy is supported in the future, the anti-HCV test should be added to the general screening test items in the population aged 40 years or older.
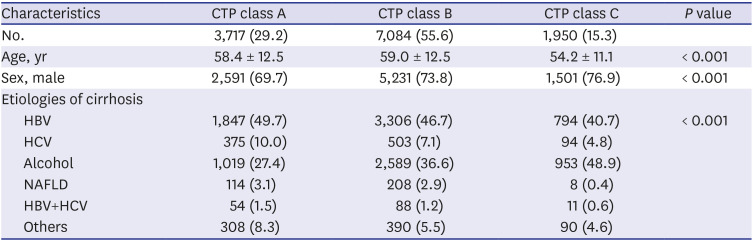
The proportion of CTP-A, which is difficult to diagnose, is increasing every year, although the ratio of CTP-C is relatively unchanged. This may be related to increased use of diagnostic tools such as CT and MRI. In recent years, it has been more common to perform abdominal ultrasound in patients with liver disease due to the provision of National Health Insurance in Korea, increasing interest in ultrasound. In patients with cirrhosis, surface nodular hyperplasia and relatively coarse echogenicity can be observed; additionally, when portal hypertension is present, ascites and spleen enlargement are common. Notably, one Korean study showed that it is useful to use the ultrasonographic scoring system to diagnose early alcoholic cirrhosis.
19 Screening abdominal ultrasound is expected to allow early diagnosis and improved prognosis of cirrhosis patients with liver disease in Korea.
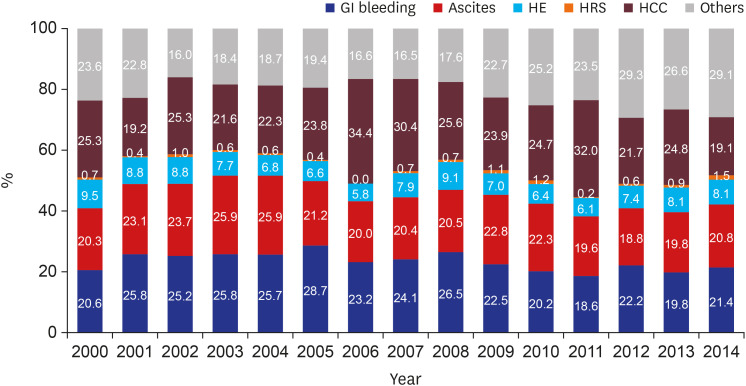
Diagnosis of HCC was divided into three groups by time period. In the period 2000-2003, HBV was the most common cause of the disease. In Korea, many studies have shown a relatively high proportion of HBV patients diagnosed with HCC in a similar period.
202122 This is because HCC commonly develops in elderly adults, and the national immunization system with HBV was applied in 1995 but did not influence the occurrence of HCC. A recent study showed that antiviral treatment with nucleoside/tide analogs has significantly reduced the risk of HCC in HBV patients.
23 However, paradoxically, one retrospective study has suggested that antiviral therapy against HBV infection has reduced liver disease mortality markedly, which may prolong life expectancy and increase the number of patients at risk of developing HCC, leading to a growing burden of HCC in an HBV-endemic population.
24 Similarly, our study showed a noticeable increase in HCC development among cirrhotic patients in the three cohort groups analyzed. One reason for this is the increased life expectancy of chronic hepatitis and cirrhosis patients, allowing increased likelihood to develop HCC. In addition, increasing awareness of HCC over time or use of diagnostic tools such as MRI and CT might have recently led to an increase in the rate of HCC diagnosis. In our study of HCC sub-analysis, patients of CTP class A (31.2%) had higher HCC incidence rate than those of CTP class C (14.3%). In addition to early diagnosis of CTP class A patients, patients with CTP class C might have shorter survival due to cirrhosis complication such as variceal bleeding, ascites, and hepatic encephalopathy. Within the limitations of our retrospective study, we were able to obtain patient survival data for a total of 5,076 patients. Of these, 616 (73.5%) of 837 expired patients with CTP class C died of cirrhosis complications before HCC was diagnosed, which suggests a higher mortality rate than that of CTP class A (47.3%). Finally, our finding that virus (HBV, HCV)-related cirrhosis had greater risk of HCC than alcohol or NAFLD cirrhosis suggests that hepatitis B and C viruses themselves have a direct carcinogenic effect.
252627
This study has many limitations due to its retrospective nature. Although the number of patients was high, the characteristics at baseline were different between the groups and were difficult to control. In addition, the results are limited by definitions of cirrhosis based on KCD-6 codes derived from medical records. These definitions of cirrhosis have been extensively validated and used in research,
2428 and diagnosis of cirrhosis in this study was consistent with previous studies based on APRI, other serologic tests and imaging tests, which are easy to confirm from medical records. However, our definition might have resulted in some patients being diagnosed with cirrhosis at a local medical clinic; therefore, our study might overestimate incidence of cirrhosis. The other limitation of our study is the lack of biopsy. It is likely that early cirrhosis patients with preserved liver function and no clinical symptoms were omitted based on our definition. Hence, we likely underestimated the true prevalence of cirrhosis. Such cases of early cirrhosis are difficult to diagnose in the absence of a liver biopsy, which is not performed in most patients. Finally, we could not follow all patients until death because patients did not visit each hospital after diagnosis of cirrhosis or were transferred to primary and secondary hospitals. Hence, we were not able to further analyze competing risk factors and mortality of cirrhosis patients.
In conclusion, although this study confirmed a recent decreasing trends in cirrhosis, the burden of cirrhosis remains in Korea and is associated with increasing morbidity and mortality. HBV, alcohol, HCV, and NAFLD are common cirrhosis etiologies. Our findings can serve to guide future public health strategies. Recently, viral hepatitis with HBV and HCV can be treated with antiviral agent. Targeted screening for at-risk patients will facilitate early detection of liver diseases allowing effective intervention and may have an impact on the development of cirrhosis and its complications. Early diagnosis of cirrhosis patients with active surveillance will improve survival of patients as well. In addition, the increase of alcohol-induced cirrhosis is entirely preventable through appropriate education and the law. Public health strategies focusing on risk factors such as excessive alcohol use and obesity are required to reduce the impact of non-viral liver disease.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download