Valproate (VPA) and antipsychotics (APs) are mood-stabilizing agents used to relieve mood irritability and aggression.
1 These drugs are also often used in combination therapies.
2 Attention deficit hyperactivity disorder (ADHD) medications can be useful for improving impulsivity and hyperactivity in children with mood irritability.
3 Among children with ADHD whose chronic aggressive behavior is refractory to optimized stimulant treatment, the addition of VPA is often used to decrease aggression and irritability.
4
Although increased risk of neutropenia in monotherapy with VPA and APs has been well documented
56; however, little is known about neutropenia in therapies combining ADHD medication with VPA or APs.
7
Recently, a number of cases of neutropenia have been observed in pediatric inpatients at the National Center for Mental Health (NCMH) in which patients were treated with a combination of ADHD medication, VPA, and APs, and which were carefully observed due to the risk of hematologic toxicity with this combination. The purpose of the current study was to evaluate the incidence of neutropenia in combination treatments of VPA, APs, and ADHD medication.
We conducted this study through retrospective review of the medical records of pediatric psychiatric inpatients at the NCMH treated with mono- or combination-therapies of VPA, APs, and ADHD medication at any time during their hospitalization. We included a total of 231 patients during the period from January 1, 2014 to February 19, 2018.
Subjects were excluded if they had previous history of neutropenia or hematologic abnormalities. Subjects were included who were treated with APs, VPA, ADHD medication (methylphenidate, atomoxetine), singly or in combination, for a minimum of 7 days. At the time of admission, a routine laboratory exam including a complete blood cell (CBC) count with differential that contained white blood cell (WBC) and absolute neutrophil count (ANC) values was conducted for each patient. The NCMH guidelines for VPA prescription require a CBC with differential before the medication is initiated as well as 1–2 weeks after initiation and at each subsequent dose increase. Subjects treated with VPA must have had at least one follow-up WBC count and ANC obtained at least 14 days after initiation of the study medication.
We divided subjects into four groups according to drug history: APs monotherapy group (AP group), VPA–AP combination group (VPA + AP group), AP–ADHD medication combination group (AP + ADHD group), and VPA–AP–ADHD medication combination group (VPA + AP + ADHD group). We excluded subjects treated with either concomitant carbamazepine or clozapine due to the boxed warning regarding potentially severe hematologic events.
The primary outcome measure is the incidence of neutropenia defined as ANC < 2,000 cells/μL. We used the Common Toxicity Criteria (CTC), version 2.0,
8 which is a standardized system for assessing the severity of adverse drug reactions, to evaluate the severity of neutropenia. The grades ranged from 0 to 5: grade 0 for no adverse events, or laboratory values within normal limits; grade 1 for 1,500 ≤ ANC < 2,000; grade 2 for 1,000 ≤ ANC < 1,500; grade 3 for 500 ≤ ANC < 1,000; grade 4 for ANC < 500 cells/μL; and grade 5 for death related to an adverse event. The probability of adverse reactions secondary to the medication was evaluated using the Naranjo probability scale.
9 Total scores > 8 indicate highly probable, 5–8 probable, 1–4 possible, and < 1 doubtful for a causal relationship between the medication and adverse reactions.
To compare demographic and clinical characteristics of the groups, we used χ2 tests for categorical variables and analysis of variance for continuous variables. To compare the factors affecting the occurrence of neutropenia, significant variables in the univariate analysis were included as independent variables in the logistic regression model, with the dependent variable being occurrence of neutropenia. The odds ratio (OR) was calculated for 95% confidence intervals (CIs). Analyses were executed using R statistical significance (R Foundation, Vienna, Austria) was defined as a P value < 0.05.
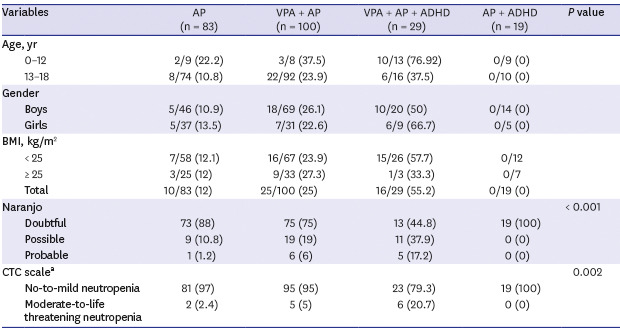
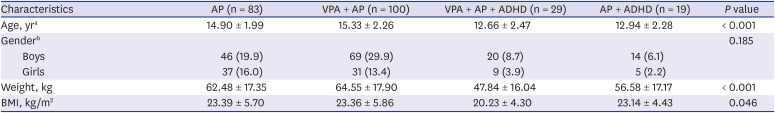
Of the 231 subjects, 83 subjects were in the AP group, 100 were in the VPA+AP group, 19 were in the AP + ADHD group, and 29 were in the VPA + AP + ADHD group. The group mean ages, body weights, and body mass indices (BMIs) were significantly different among groups, and gender differences were not significant (
Table 1).
Table 1
Demographic characteristics
|
Characteristics |
AP (n = 83) |
VPA + AP (n = 100) |
VPA + AP + ADHD (n = 29) |
AP + ADHD (n = 19) |
P value |
|
Age, yra
|
14.90 ± 1.99 |
15.33 ± 2.26 |
12.66 ± 2.47 |
12.94 ± 2.28 |
< 0.001 |
|
Genderb
|
|
|
|
|
0.185 |
|
Boys |
46 (19.9) |
69 (29.9) |
20 (8.7) |
14 (6.1) |
|
Girls |
37 (16.0) |
31 (13.4) |
9 (3.9) |
5 (2.2) |
|
Weight, kg |
62.48 ± 17.35 |
64.55 ± 17.90 |
47.84 ± 16.04 |
56.58 ± 17.17 |
< 0.001 |
|
BMI, kg/m2
|
23.39 ± 5.70 |
23.36 ± 5.86 |
20.23 ± 4.30 |
23.14 ± 4.43 |
0.046 |

Table 2 compares the age, gender, and BMI according to occurrence of neutropenia. The neutropenia group was younger and had lower BMI than the control group (
P < 0.001). Girls had a higher risk of neutropenia than boys (
P < 0.001). The incidences of neutropenia were 55.2%, 25%, and 12% in the VPA + AP + ADHD group, VPA + AP group, and AP group, respectively (
P < 0.001). Further analysis of the VPA + AP + ADHD group showed no significant difference in the incidence of neutropenia between subgroups using methylphenidate versus atomoxetine (50% vs. 50%).
Table 2
Incidence of neutropenia
|
Variables |
AP (n = 83) |
VPA + AP (n = 100) |
VPA + AP + ADHD (n = 29) |
AP + ADHD (n = 19) |
P value |
|
Age, yr |
|
|
|
|
|
|
0–12 |
2/9 (22.2) |
3/8 (37.5) |
10/13 (76.92) |
0/9 (0) |
|
|
13–18 |
8/74 (10.8) |
22/92 (23.9) |
6/16 (37.5) |
0/10 (0) |
|
|
Gender |
|
|
|
|
|
|
Boys |
5/46 (10.9) |
18/69 (26.1) |
10/20 (50) |
0/14 (0) |
|
|
Girls |
5/37 (13.5) |
7/31 (22.6) |
6/9 (66.7) |
0/5 (0) |
|
|
BMI, kg/m2
|
|
|
|
|
|
|
< 25 |
7/58 (12.1) |
16/67 (23.9) |
15/26 (57.7) |
0/12 |
|
|
≥ 25 |
3/25 (12) |
9/33 (27.3) |
1/3 (33.3) |
0/7 |
|
|
Total |
10/83 (12) |
25/100 (25) |
16/29 (55.2) |
0/19 (0) |
|
|
Naranjo |
|
|
|
|
< 0.001 |
|
Doubtful |
73 (88) |
75 (75) |
13 (44.8) |
19 (100) |
|
Possible |
9 (10.8) |
19 (19) |
11 (37.9) |
0 (0) |
|
Probable |
1 (1.2) |
6 (6) |
5 (17.2) |
0 (0) |
|
CTC scalea
|
|
|
|
|
0.002 |
|
No-to-mild neutropenia |
81 (97) |
95 (95) |
23 (79.3) |
19 (100) |
|
Moderate-to-life threatening neutropenia |
2 (2.4) |
5 (5) |
6 (20.7) |
0 (0) |

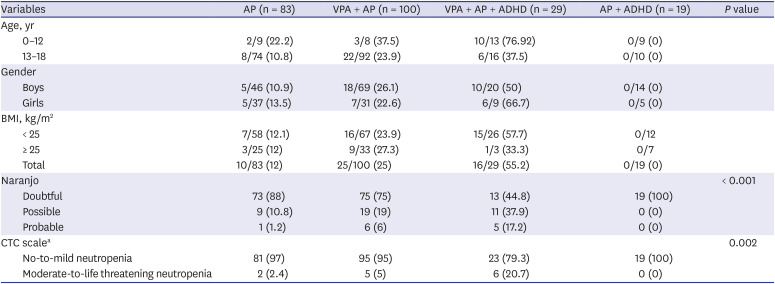
In the AP group, 10 of the 83 subjects developed neutropenia. According to the ANC CTC severity score, 8 subjects had mild and 2 had moderate neutropenia. In the VPA + AP group, 25 of the 100 subjects developed neutropenia: 20 developed mild and 5 developed moderate neutropenia. In the VPA + AP + ADHD group, 16 of the 29 patients developed neutropenia: 10 subjects had mild, 4 had moderate, 1 had severe, and 1 had life-threatening neutropenia.
We used the Naranjo probability scale to assess the probability that the drugs induced neutropenia. In the AP group, 9 of 10 (90%) case of neutropenia scored as possible that they were caused by the drug, and 1 of 10 (10%) incidents scored as probable. In the VPA + AP group, 19 of 25 (76%) cases scored possible, and 6 of 25 (24%) cases scored probable. In the VPA + AP + ADHD group, 11 of 29 (37.9%) cases scored possible and 5 of 29 (17.2%) scored probable. There were significant differences of ANC CTC severity scores and Naranjo probability scores among drug classes.
The logistic regression analysis including presence of neutropenia as an independent variable and age, gender, and BMI as covariates showed that those in the VPA + AP group were 2.59-fold (95% CI, 1.15–5.87; P = 0.02) more likely to develop neutropenia than those in the AP group, and those in the VPA + AP + ADHD group were 6.43-fold (95% CI, 2.26–18.26; P < 0.001) more likely to develop neutropenia than those in the AP group. However, the AP + ADHD group showed no significant difference in the risk of neutropenia compared to the AP group. In the additional subgroup analysis by age group, the risk of neutropenia in the group over 12 years of age was increased for the VPA + AP group (OR, 2.68; 95% CI, 1.11–6.51; P = 0.03) and VPA + AP + ADHD group (OR, 5.36; 95% CI, 1.42–20.31; P = 0.01), when compared with the AP group. In the group under 12 years of age, the risk of neutropenia was not different among drug class after adjusting for age, gender and BMI.
This study is the first to explore the incidence of neutropenia for treatment with AP, VPA, and ADHD medication, singly or in combination, in children. Compared with those in the AP group, those in the VPA + AP group were more than twice as likely to experience neutropenia, and the VPA + AP + ADHD group had a 55.2% incidence of neutropenia. These findings were further supported by the results of our analysis based on the CTC severity scale and the Naranjo probability scale. The VPA + AP + ADHD group showed higher neutropenia severity than did the VPA + AP group and the AP group.
The result of our study should be interpreted with caution. Due to the insufficient number of VPA + AP + ADHD group, we did not conclude that ADHD medication increases the risk of neutropenia. In addition, the possible mechanism of how ADHD medication increases the risk of neutropenia is not suggested in this study. However, it can be assumed that the dopamine and norepinephrine enhancement of ADHD medication probably caused neutropenia directly, or acted as a synergistic effect in the process of VPA and APs inducing neutropenia. Prior study has reported that dopamine induces apoptosis of human neutrophils in vitro.
10 Moreover, animal study demonstrated that noradrenaline and dopamine affect regulation of hematopoiesis.
11 Future research with larger sample will be needed to examine the incidence of neutropenia and how ADHD medication affects the neutropenia.
A study limitation is its relatively small sample size. However, to our knowledge, it is the largest sample size yet used to examine the incidence of neutropenia with APs and VPA. In addition, this study is valuable in its focus on child, unlike previous studies. Another study limitation is that factors known to affect neutropenia (e.g., nutrition, malignancy, radiation exposures) are not controlled as covariates. And the definition of drug induced neutropenia (ANC < 2,000/μL), we used was different from the criteria (ANC < 1,500/μL) commonly used medically in clinical situations.
12 Most of the neutropenia cases in our study were benign and self-remitting. Finally, the study is limited by the lack of information on the medication use such as dosage, duration and types of APs.
Ethics Statement
The study protocol was approved by the Institutional Review Board (IRB) of the National Center for Mental Health (IRB No. 116271-2018-27).
Go to :