INTRODUCTION
The cytokine storm syndrome, which rapidly progress to acute respiratory distress syndrome (ARDS), shock, and multiorgan dysfunction, has been observed in a subgroup of patients with severe coronavirus disease 2019 (COVID-19).
12 Several therapeutic measures, including steroids and cytokine blockade, have been suggested.
3 We present the case of a COVID-19 patient whose condition rapidly deteriorated with hyperpyrexia but was successfully managed with therapeutic temperature modulation (TTM).
CASE DESCRIPTION
On March 5, 2020, a-58-year-old woman was transferred to our intensive care unit (ICU) due to acute hypoxemic respiratory failure. The patient was previously diagnosed with COVID-19 on a screening test for throat discomfort 12 days earlier. The patient lived in Daegu, the epicenter of COVID-19 in Korea, and had acquired the infection through community spread. Seven days before ICU admission, the patient was diagnosed with pneumonia and started lopinavir/ritonavir 400 mg/100 mg tablets twice a day and supplemental oxygen was started 3 days ago. Vital signs on presentation to the ICU were: blood pressure 145/90 mmHg, pulse rate 90/min, respiratory rate 32/min, and body temperature 36.9°C. Arterial blood gas analysis showed pH 7.383, partial pressure of carbon dioxide (pCO
2) 33.5 mmHg, partial pressure of arterial oxygen (PaO
2) 83.9 mmHg, and bicarbonate (HCO
3-) 19.5 mmol/L on high-flow nasal cannula (fraction of inspired oxygen [FiO
2], 0.95; flow rate, 50 L/min). The patient was intubated and placed on mechanical ventilation with elaborate lung-protective strategies.
4 The PaO
2 improved to 229.1 mmHg on FiO
2 0.9, and we initiated intravenous methylprednisolone 60 mg. A chest radiograph showed bilateral, multifocal, patchy consolidations in the lungs (
Fig. 1). The C-reactive protein level was 22.9 mg/dL. Nasopharyngeal and oropharyngeal swabs as well as sputum specimens were obtained for retesting at our hospital and reported positive for infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay.
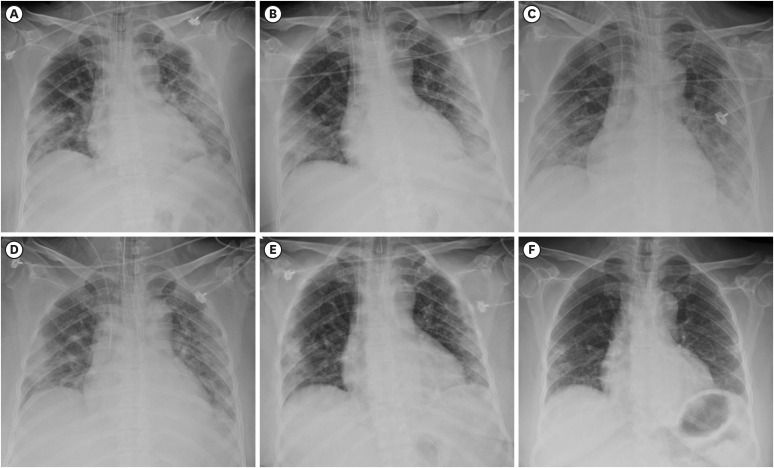
Fig. 1
Chest radiographs on (A) Day 1, (B) Day 2, (C) Day 3, (D) Day 5, (E) Day 8, and (F) Day 22 of hospitalization.
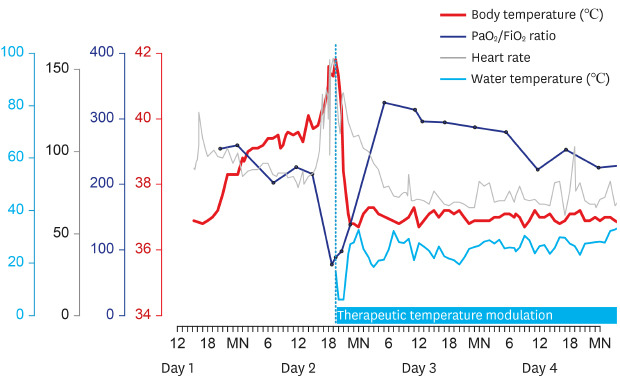
From the evening on Day 1 of hospitalization, the patient's body temperature began to increase, reached approximately 40°C on Day 2, and was followed by tachycardia and desaturation (
Fig. 2). The fever was refractory to repeated administration of antipyretics. The body temperature increased up to 41.6°C, heart rate to 156 per min, and oxygen saturation decreased to 88% on FiO
2 of 1.0. Nitric oxide (NO) inhalation was initiated and raised to 40 ppm, and injection of methylprednisolone 60 mg was repeated. However, arterial blood gas analysis showed PaO
2 of 77.6 mmHg, and the troponin I level increased to 6.22 ng/mL. Considering extracorporeal membrane oxygenation (ECMO) as the last resort for treatment, we applied a surface cooling device (Arctic Sun®) with a target temperature of 37°C. From an initial core temperature of 41.8°C, the target temperature was rapidly attained within 4.5 hours. PaO
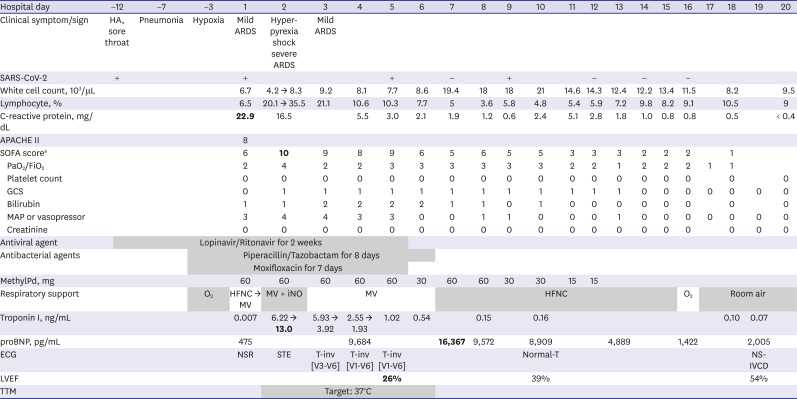
2 was markedly improved to 318.2 mmHg, and tachycardia resolved after a few hours. The norepinephrine requirement that had increased up to 16 µg/min during the shock phase was tapered off. Echocardiography on Day 4 showed mid-to-apical-segment akinesia and depressed left ventricular systolic function with an ejection fraction of 26%. The patient was extubated on Day 6 and TTM was stopped on Day 9. Other treatment details including antibiotics and steroid use are summarized in
Table 1. On Day 12, SARS-CoV-2 RT PCR results converted negatively in all nasopharyngeal, oropharyngeal, and sputum specimens. Supplemental oxygen was discontinued on Day 16, and the patient's cardiac function was confirmed to have recovered on Day 19 with an ejection fraction of 54%. A coronary computed tomographic angiography was also normal. A chest radiograph on Day 22 showed considerable improvement compared to the previous ones (
Fig. 1).
Fig. 2
Body temperature, the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (PaO2/FiO2), and heart rate during hyperpyrexia with subsequent changes indicating the effect of therapeutic temperature modulation.
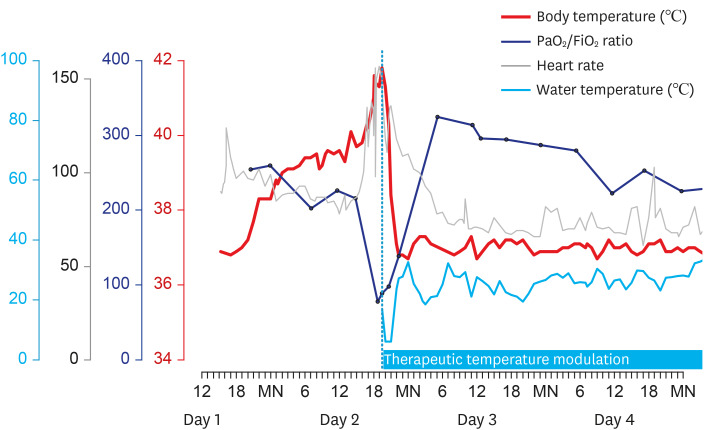
Table 1
Clinical course, laboratory findings, and management of the patient
|
Hospital day |
−12 |
−7 |
−3 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
|
Clinical symptom/sign |
HA, sore throat |
Pneumonia |
Hypoxia |
Mild ARDS |
Hyper-pyrexia shock severe ARDS |
Mild ARDS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SARS-CoV-2 |
+ |
|
|
+ |
|
|
|
+ |
|
− |
|
+ |
|
|
− |
|
− |
|
− |
|
|
|
|
|
White cell count, 103/μL |
|
|
|
6.7 |
4.2 → 8.3 |
9.2 |
8.1 |
7.7 |
8.6 |
19.4 |
18 |
18 |
21 |
14.6 |
14.3 |
12.4 |
12.2 |
13.4 |
11.5 |
|
8.2 |
|
9.5 |
|
Lymphocyte, % |
|
|
|
6.5 |
20.1 → 35.5 |
21.1 |
10.6 |
10.3 |
7.7 |
5 |
3.6 |
5.8 |
4.8 |
5.4 |
5.9 |
7.2 |
9.8 |
8.2 |
9.1 |
|
10.5 |
|
9 |
|
C-reactive protein, mg/dL |
|
|
|
22.9
|
16.5 |
|
5.5 |
3.0 |
2.1 |
1.9 |
1.2 |
0.6 |
2.4 |
5.1 |
2.8 |
1.8 |
1.0 |
0.8 |
0.8 |
|
0.5 |
|
< 0.4 |
|
APACHE II |
|
|
|
8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SOFA scorea
|
|
|
|
6 |
10
|
9 |
8 |
9 |
6 |
5 |
6 |
5 |
5 |
3 |
3 |
3 |
2 |
2 |
2 |
|
1 |
|
|
|
PaO2/FiO2
|
|
|
|
2 |
4 |
2 |
2 |
3 |
3 |
3 |
3 |
3 |
3 |
2 |
2 |
1 |
2 |
2 |
2 |
1 |
1 |
|
|
|
Platelet count |
|
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0 |
|
0 |
|
GCS |
|
|
|
0 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Bilirubin |
|
|
|
1 |
1 |
2 |
2 |
2 |
2 |
1 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0 |
|
0 |
|
MAP or vasopressor |
|
|
|
3 |
4 |
4 |
3 |
3 |
0 |
0 |
1 |
1 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Creatinine |
|
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0 |
0 |
0 |
|
Antiviral agent |
|
Lopinavir/Ritonavir for 2 weeks |
|
|
Antibacterial agents |
|
Piperacillin/Tazobactam for 8 days |
|
|
Moxifloxacin for 7 days |
|
|
MethylPd, mg |
|
|
|
60 |
60 |
60 |
60 |
60 |
30 |
60 |
60 |
30 |
30 |
15 |
15 |
|
|
|
|
|
|
|
|
|
Respiratory support |
|
|
O2
|
HFNC → MV |
MV + iNO |
MV |
HFNC |
O2
|
Room air |
|
Troponin I, ng/mL |
|
|
|
0.007 |
6.22 → 13.0
|
5.93 → 3.92 |
2.55 → 1.93 |
1.02 |
0.54 |
|
0.15 |
|
0.16 |
|
|
|
|
|
|
|
0.10 |
0.07 |
|
|
proBNP, pg/mL |
|
|
|
475 |
|
|
9,684 |
|
|
16,367
|
9,572 |
|
8,909 |
|
|
4,889 |
|
|
1,422 |
|
|
2,005 |
|
|
ECG |
|
|
|
NSR |
STE |
T-inv [V3-V6] |
T-inv [V1-V6] |
T-inv [V1-V6] |
|
|
|
|
Normal-T |
|
|
|
|
|
|
|
|
NS-IVCD |
|
|
LVEF |
|
|
|
|
|
|
|
26%
|
|
|
|
|
39% |
|
|
|
|
|
|
|
|
54% |
|
|
TTM |
|
Target: 37°C |
|
DISCUSSION
The patient presented with mild ARDS but rapidly progressed to severe ARDS and shock with hyperpyrexia, probably due to a hyperinflammatory syndrome. However, the patient demonstrated rapid improvement in oxygenation and shock after the initiation of TTM. Pyrexia confers a metabolic cost, which increases oxygen consumption by 10% per degree Celsius rise in temperature.
5 Therefore, rapid reduction in core temperature by 5°C successfully offloaded the patient's failing cardiorespiratory system. The hyperinflammatory response and hyperpyrexia may have a reciprocal synergism, wherein uncontrolled inflammation promotes thermogenesis and vice versa, and this vicious cycle may have been forcibly terminated by TTM.
6
The pandemic caused by SARS-CoV-2 infection has necessitated several management strategies, although many have not been concretely established as conferring a patient benefit. ECMO can serve as a life-saving rescue therapy, although concerns exist about potential adverse effects of ECMO therapy for patients with COVID-19.
7 The therapeutic temperature modulation may safely be applied in a specific group of patients with cytokine storm syndrome and hyperpyrexia. If efficacy and safety can be demonstrated through further studies, targeted therapeutic modulation will reduce the number of patients requiring ECMO in the global medical resource shortage. Despite the potential contribution of pyrexia to host defense, the dramatic response to TTM in this patient suggests that the severe metabolic stress induced by hyperpyrexia may be considerably more harmful than beneficial in the hyperinflammatory stage of SARS-CoV-2 infection.
Ethics statement
The Institutional Review Board of Seoul National University Bundang Hospital approved the publication of this report, and written informed consent was waived (B-2005-611-701).







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download