This article has been
cited by other articles in ScienceCentral.
Abstract
Stereotactic cardiac radiation for ablation (radioablation) of life-threatening ventricular arrhythmia was recently introduced into clinical practice. A 76-year-old male patient with apical hypertrophic cardiomyopathy at burnout stage, who received defibrillator implantation for the secondary prevention of sudden arrhythmic death, was admitted for repeated defibrillator therapy. Radiofrequency catheter ablation was unsuccessful due to the induction of ventricular fibrillation (VF) and hemodynamically unstable sustained monomorphic ventricular tachycardia (VT). However, intracardiac activation mapping for the induced VT revealed the earliest ventricular activation at the apical aneurysm. Radioablation was performed to control VT and VF storm refractory to antiarrhythmic drug therapy. A total of 24 Gray was radiated, divided into three fractions around the apical aneurysm. The onset of electrical modulation was instantaneous and the antiarrhythmic effect was maintained for at least 6 months without significant radiation toxicities. This case suggests that radioablation may be considered as a rescue therapy for VT and VF storm refractory to other treatment modalities.
Keywords: Radiation, Ablation, Ventricular Tachycardia, Cardiomyopathy
INTRODUCTION
Stereotactic cardiac radiation for ablation (radioablation) for life-threatening ventricular arrhythmia was introduced into clinical practice in 2017.
1 In a case series and a small observational study, radioablation was shown to successfully reduce the burden of ventricular arrhythmias with acceptable short term toxicities, by combining electrocardiographic imaging and stereotactic body radiation techniques.
23 However, clarity on the indication and proper methodology for radioablation have not yet been clearly established. We recently performed inevitable radioablation to control ventricular tachycardia (VT) and ventricular fibrillation (VF) storm in a patient with apical hypertrophic cardiomyopathy at burnout stage and an implantable cardioverter-defibrillator (ICD). As VT and VF storm was successfully controlled by radioablation, the patient's clinical course has been described in this case report.
CASE DESCRIPTION
A 76-year-old male patient with apical hypertrophic cardiomyopathy at burnout stage and an ICD was admitted for recurrent VT in December 1, 2018. The patient underwent ICD implantation for out-of-hospital cardiac arrest three years prior. Although the patient had been taking sotalol 240 mg/day, the number of fast VT episodes treated by anti-tachycardia pacing increased rapidly for several months, and the patient eventually lost consciousness during ICD charging for shock delivery (
Fig. 1). The patient was admitted for radiofrequency catheter ablation (RFCA).
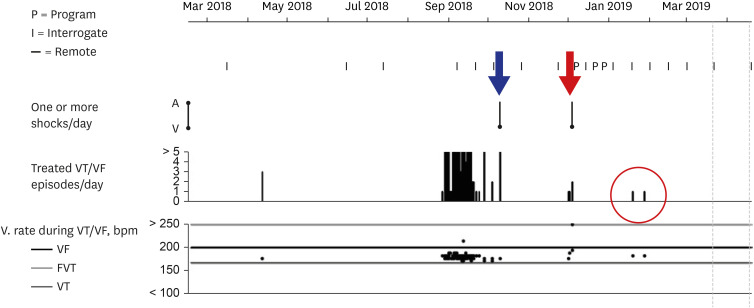
Fig. 1
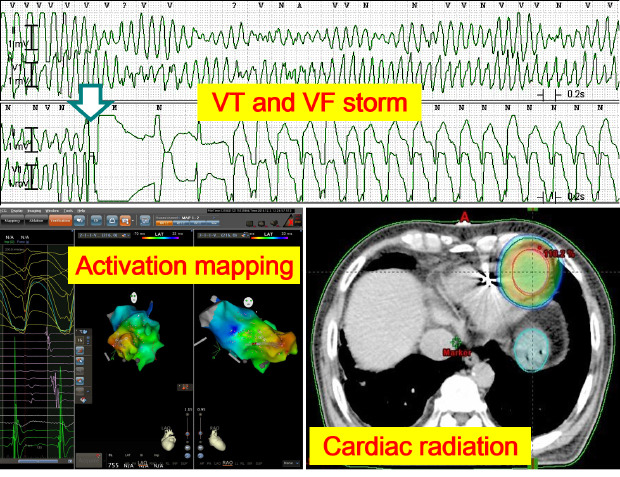
Report of VT and VF episodes recorded by the patient's ICD.
ICD interrogation showed that the number of treated VT episodes/day requiring anti-tachycardia pacing had increased remarkably from September 2018. When ICD shock was delivered in October 2018 (blue arrow), the patient lost consciousness during ICD charging. Because treated VT episodes/day increased again in December 2018, the patient was admitted for ablation. Stereotactic cardiac radiation was performed on 5 December 2018 to control VT and VF storm (red arrow) in which ICD shocks were delivered 7 times for 24 hours. Although sustained VT requiring anti-tachycardia pacing recurred two times at 6 and 8 weeks (red circle) while reducing the doses of antiarrhythmic agents, ICD shock was no longer delivered.
VT = ventricular tachycardia, VF = ventricular fibrillation, ICD = implantable cardioverter-defibrillator.
Intracardiac mapping was performed using a 3-dimensional navigation system (CARTO® 3 System, Biosense Webster, Diamond Bar, CA, USA). During a baseline electrophysiologic study, VF was induced by right ventricular pacing at a cycle length of 210 ms and should be terminated by external defibrillation at 150 J. To avoid the risk of VF re-induction, we decided to perform substrate modulation. However, during geometric mapping of the left ventricle (LV), a sustained monomorphic VT of 280 bpm was induced by ventricular premature beats. Electrocardiographic features of both clinically documented and induced VTs suggested that they came from the same arrhythmogenic substrates located in the apical aneurysm (
Fig. 2A and B). Activation mapping for the induced VT using a multi-electrode catheter showed the earliest ventricular activation at the septal side of the apical aneurysm with apical-to-basal propagation (
Fig. 3A and B). However, the patient's blood pressure dropped to 60/30 mmHg within 4 minutes after induction of the VT, which should be terminated by repeated external cardioversion at 150 J. As the patient refused further ablation, the procedures should be finished after infusion of 300 mg amiodarone to prevent recurrence of hemodynamically unstable VT or VF. However, fast VT and VF requiring ICD shock therapy recurred the next day (
Fig. 2C). Although amiodarone at a dose of 20 mg/kg/day was continuously infused in combination with oral flecainide 200 mg/day, ICD shocks were delivered seven times over 24 hours. Because the patient refused any invasive procedures including extracorporeal membrane oxygenation-supported RFCA, cardiac sympathetic denervation, and heart transplantation, we decided to perform stereotactic cardiac radiation for ablation as a rescue therapy after informed consent.
Fig. 2
VT and VF electrocardiographic findings.
(A) Sustained monomorphic VT (cycle length, 240 ms; QRS duration, 120 ms) documented 3 years prior to ICD implantation showed a right bundle branch block configuration in the V1 lead, cardiac axis to no man's land, and cardiac transition in the V3 lead, suggesting a left ventricular apicolateral origin. (B) Sustained monomorphic VT (cycle length, 240 ms; QRS duration, 120 ms) induced during the electrophysiologic study showed left bundle branch block configuration in the V1 lead, cardiac axis to no man's land, and negative precordial concordance, which suggested a right ventricular apical or left ventricular apical septal origin. (C) Telemetry recordings showed a recurrence of fast VT and VF, which should be terminated by ICD shock (red arrow).
VT = ventricular tachycardia, VF = ventricular fibrillation, ICD = implantable cardioverter-defibrillator.
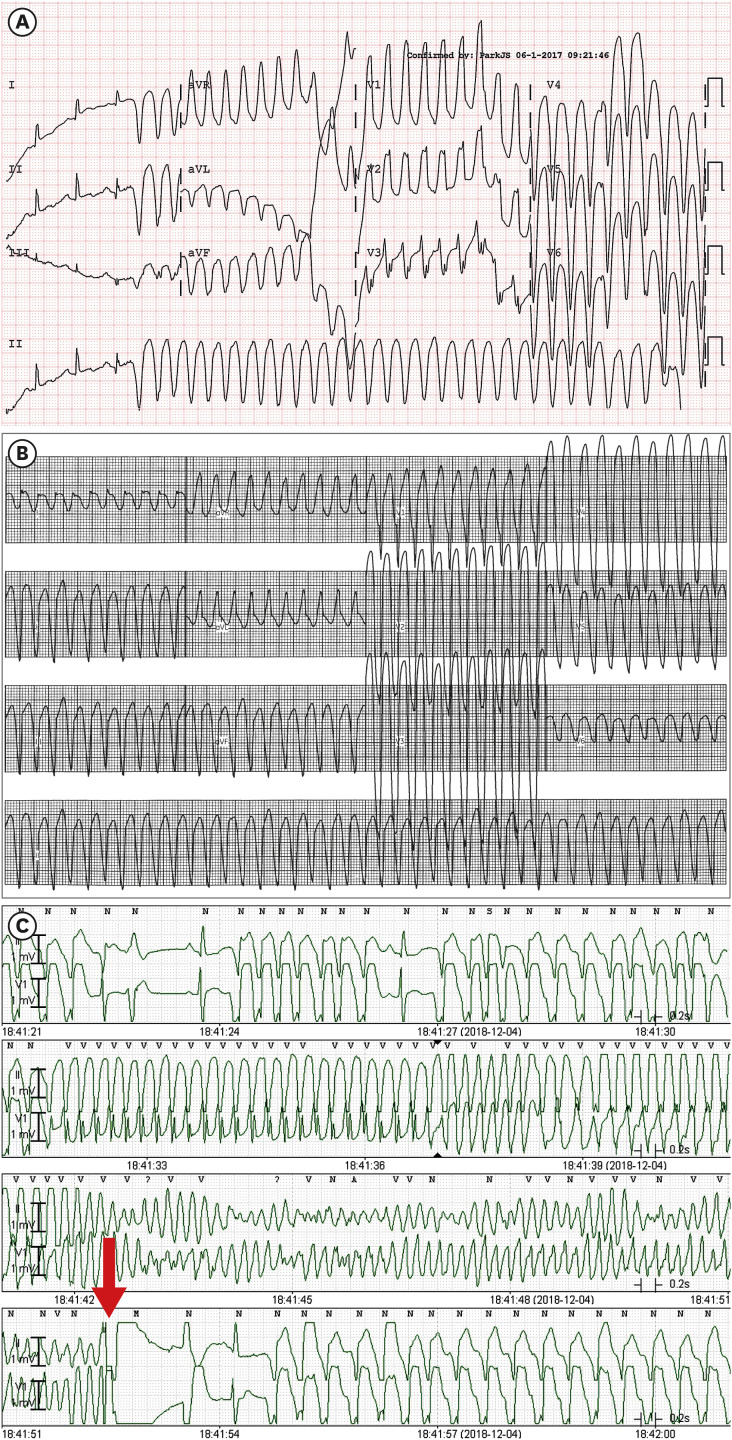
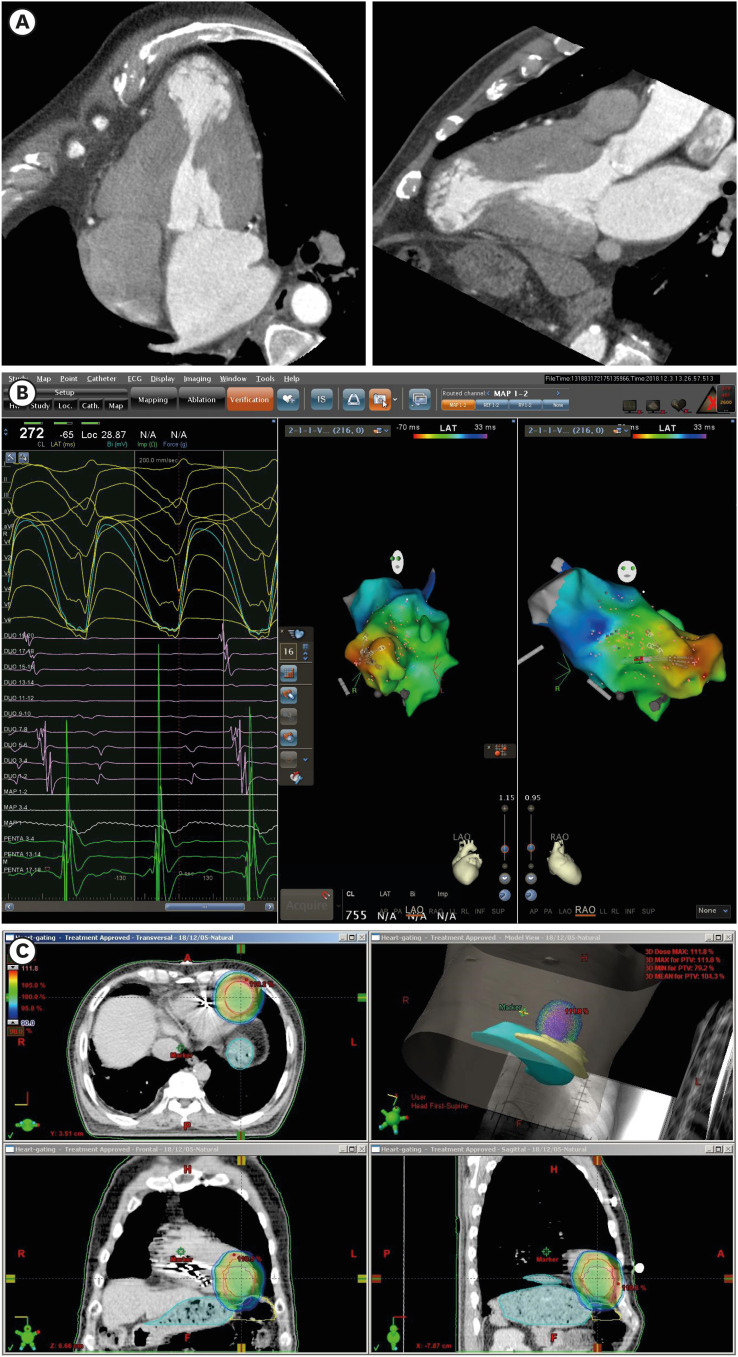
Fig. 3
Electroanatomic targeting of arrhythmogenic substrates for cardiac radioablation.
(A) Cardiac multidetector computed tomography images reveal aneurysmal dilation of the cardiac apex compatible with apical hypertrophic cardiomyopathy at burnout stage. (B) Activation mapping of the sustained monomorphic ventricular tachycardia using a multi-electrode mapping catheter and three-dimensional navigation system revealed that ventricular activation (red color) began in the septal side of the LV apical aneurysm and propagated to the LV base (blue color). (C) The gross tumor volume for stereotactic cardiac radiation was defined as the LV apical aneurysm and surrounding myocardium with 1 cm extension from the aneurysmal border. The proximal end of the defibrillator shock coil was used as a radiologic marker for the patient setup.
LV = left ventricle.
Radioablation was administered under respiratory gating on the first, second, and fifth days with a 6 megavolt X-ray using a linear accelerator (Clinac iX
™, Varian Medical Systems, Palo Alto, CA, USA). The gross tumor volume was defined as the LV aneurysm and surrounding myocardium with 1-cm extension from the aneurysmal border to comprise the potential arrhythmogenic substrates and electrical exits of VT and VF. The clinical target volume was generated by combining the gross tumor volume in free breathing and end-expiratory states. The planning target volume consisted of the clinical target volume plus a 1-cm margin in all directions (
Fig. 3C). Initially 25–35 Gy in a fraction was planned similarly to the radioablation protocols used in prior clinical studies,
23 but 24 Gy in three fractions was adopted due to the excessive gastric irradiation in the single fraction.
4
Immediately after the initiation of radioablation, the daily burden of total ventricular arrhythmias and treated VT/VF episodes began to decrease rapidly (
Fig. 4A and B). The patient was discharged after two weeks on oral amiodarone 400 mg/day and flecainide 200 mg/day. During the follow-up duration of 6 months, although anti-tachycardia pacing was performed twice, ICD shocks were no longer required (
Fig. 1). The LV ejection fraction remained unchanged at 62%–63% after 6 months. Although mild pulmonary fibrosis was noted on chest X-ray, significant cardiac or extracardiac tissue toxicities did not appear.
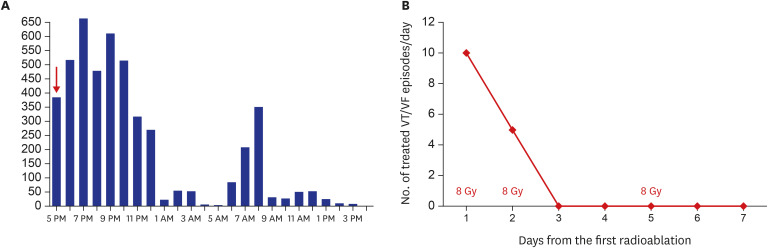
Fig. 4
Response to cardiac radioablation.
(A) The first stereotactic cardiac radiation was performed at 5 pm (red arrow). The burden of couplets of the ventricular premature beat recorded by 24-hour ambulatory electrocardiographic monitoring decreased remarkably over the initial 24 hours. (B) A total of 24 Gy was delivered to the planned target volume on the first, second, and fifth days. The number of treated VT/VF episodes/day began to decrease immediately after delivery of the first radiation dose. After delivery of 16 Gy, VT/VF episodes requiring anti-tachycardia pacing or shock therapy did not recur.
Gy = Gray, VT = ventricular tachycardia, VF = ventricular fibrillation.
Ethics statement
Written informed consent for cardiac radioablation and publication of entire treatment process was obtained from the patient. All images are published under agreement of the patient. Appropriateness of treatment process was reviewed and publication was approved by the Institutional Review Board of the Dong-A University Hospital (Approval No. DAUHIRB-EXP-19-206).
DISCUSSION
Animal studies have shown that cardiac radiation of 25–55 Gray induces myocardial tissue damage with fibrosis and reduces conduction velocity similar to that of RFCA.
5678 The efficacy and safety of radioablation for ventricular tachyarrhythmia has been recently evaluated in human studies.
123910 Compared with conventional RFCA, radioablation is more likely to produce homogenous transmural lesions which can provide greater benefit in electrical and pathologic modulation of the arrhythmogenic substrates.
For stereotactic cardiac radioablation, prior studies used electrocardiographic imaging technology which, by combining cardiac multi-detector computed tomography and 256-electrode surface electrocardiography, can provide information about both the arrhythmogenic substrates and electrical activities of ongoing ventricular arrhythmias. However, it is not yet available in Korea and technically impossible to perform if ventricular arrhythmia is hemodynamically unstable. In the present case, we could define the potential origin of the VT and VF based on the findings of conventional cardiac imaging, electrocardiographic, and electrophysiologic studies.
Stereotactic body radiation was performed using a linear accelerator system widely available in Korea. Suppression of VT and VF storm was instantaneous and the effect of electrical modulation was maintained for at least 6 months without significant radiation toxicities. This case shows that stereotactic cardiac radioablation may be considered as a rescue therapy for scenarios involving VT and VF storm that cannot be controlled by other treatment modalities including antiarrhythmic drug therapy and RFCA.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download