1. International Society of Nephrology. ISN Global Kidney Health Atlas. Brussels: International Society of Nephrology;2017.
2. Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Health Behavior and Chronic Disease Statistics 2015 Korea National Health and Nutrition Examination Survey (KNHANES VI-3). Cheongju: Korea Centers for Disease Control and Prevention;2016.
3. Beto JA, Bansal VK. Medical nutrition therapy in chronic kidney failure: integrating clinical practice guidelines. J Am Diet Assoc. 2004; 104(3):404–409. PMID:
14993863.

4. Royal College of Physicians of London. National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians of London;2008.
5. Lee JH. Management of pre-ESRD patients. Korean J Med. 1999; 57(4):783–790.
6. Peev V, Nayer A, Contreras G. Dyslipidemia, malnutrition, inflammation, cardiovascular disease and mortality in chronic kidney disease. Curr Opin Lipidol. 2014; 25(1):54–60. PMID:
24345987.

7. Sinha AD, Agarwal R. Chronic renal disease progression: treatment strategies and potassium intake. Semin Nephrol. 2013; 33(3):290–299. PMID:
23953806.

8. Byham-Gray LD, Burrowes JD, Chertow GM. Nutrition in Kidney Disease. New York, NY: Humana Press;2014.
9. Díaz-López A, Bulló M, Basora J, Martínez-González MA, Guasch-Ferré M, Estruch R, et al. Cross-sectional associations between macronutrient intake and chronic kidney disease in a population at high cardiovascular risk. Clin Nutr. 2013; 32(4):606–612. PMID:
23141101.

10. Hsu CC, Jhang HR, Chang WT, Lin CH, Shin SJ, Hwang SJ, et al. Associations between dietary patterns and kidney function indicators in type 2 diabetes. Clin Nutr. 2014; 33(1):98–105. PMID:
23706976.

11. Lin J, Judd S, Le A, Ard J, Newsome BB, Howard G, et al. Associations of dietary fat with albuminuria and kidney dysfunction. Am J Clin Nutr. 2010; 92(4):897–904. PMID:
20702608.

12. KDOQI. National Kidney Foundation. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013; 3(1):S1–163.
13. Levey AS, Greene T, Sarnak MJ, Wang X, Beck GJ, Kusek JW, et al. Effect of dietary protein restriction on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis. 2006; 48(6):879–888. PMID:
17162142.

14. Levey AS, Greene T, Beck GJ, Caggiula AW, Kusek JW, Hunsicker LG, et al. Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? J Am Soc Nephrol. 1999; 10(11):2426–2439. PMID:
10541304.
15. Menon V, Kopple JD, Wang X, Beck GJ, Collins AJ, Kusek JW, et al. Effect of a very low-protein diet on outcomes: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis. 2009; 53(2):208–217. PMID:
18950911.

16. Jones-Burton C, Mishra SI, Fink JC, Brown J, Gossa W, Bakris GL, et al. An in-depth review of the evidence linking dietary salt intake and progression of chronic kidney disease. Am J Nephrol. 2006; 26(3):268–275. PMID:
16763384.

17. Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015; 18(3):254–262. PMID:
25807354.

18. Kopple JD. National Kidney Foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001; 37(1):Suppl 2. S66–S70. PMID:
11158865.

19. Akbulut G, Sanlıer N, Inal S, Tek NA, Oneç K, Erten Y. Daily dietary energy and macronutrient intake and anthropometric measurements of the peritoneal dialysis patients. Ren Fail. 2013; 35(1):56–61. PMID:
23101754.

20. Cupisti A, D'Alessandro C, Valeri A, Capitanini A, Meola M, Betti G, et al. Food intake and nutritional status in stable hemodialysis patients. Ren Fail. 2010; 32(1):47–54. PMID:
20113266.

21. Martins AM, Dias Rodrigues JC, de Oliveira Santin FG, Barbosa Brito FS, Bello Moreira AS, Lourenço RA, et al. Food intake assessment of elderly patients on hemodialysis. J Ren Nutr. 2015; 25(3):321–326. PMID:
25572139.

22. Rho SN, Choi YC. Assessment of nutritional status and survey of dietary habits in predialysis patients of chronic renal failure. J East Asian Soc Diet Life. 2003; 13(5):408–424.
23. National Kidney Foundation. National Kidney Foundation. KDOQI Clinical practice guideline for diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012; 60(5):850–886. PMID:
23067652.
24. Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014; 64(4):510–533. PMID:
25257325.

25. American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010; 33(Suppl 1):S11–S61. PMID:
20042772.
26. Ko GJ, Kalantar-Zadeh K, Goldstein-Fuchs J, Rhee CM. Dietary approaches in the management of diabetic patients with kidney disease. Nutrients. 2017; 9(8):824–836.

27. Whitham D. Nutrition for the prevention and treatment of chronic kidney disease in diabetes. Can J Diabetes. 2014; 38(5):344–348. PMID:
25201774.

28. Oh YS, Ann JY, Kim MH, Choe SJ, Jeong JC. A prospective study on nutritional status and nutrient intake of hemodialysis patients based on coexistence of diabetes. J Korean Diet Assoc. 2017; 23(1):1–13.
29. Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013; 84(6):1096–1107. PMID:
23698226.
30. Stratton RJ, Bircher G, Fouque D, Stenvinkel P, de Mutsert R, Engfer M, et al. Multinutrient oral supplements and tube feeding in maintenance dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2005; 46(3):387–405. PMID:
16129200.

31. Huang MC, Chen ME, Hung HC, Chen HC, Chang WT, Lee CH, et al. Inadequate energy and excess protein intakes may be associated with worsening renal function in chronic kidney disease. J Ren Nutr. 2008; 18(2):187–194. PMID:
18267211.

32. Machado AD, Anjos FS, Domingos MA, Molina MD, Marchioni DM, Benseñor IJ, et al. Dietary intake of non-dialysis chronic kidney disease patients: the PROGREDIR study. A cross-sectional study. Sao Paulo Med J. 2018; 136(3):208–215. PMID:
29924288.

33. Włodarek D, Głąbska D, Rojek-Trębicka J. Assessment of diet in chronic kidney disease female predialysis patients. Ann Agric Environ Med. 2014; 21(4):829–834. PMID:
25528929.

34. Otoda T, Kanasaki K, Koya D. Low-protein diet for diabetic nephropathy. Curr Diab Rep. 2014; 14(9):523–532. PMID:
24986448.

35. Kovesdy CP, George SM, Anderson JE, Kalantar-Zadeh K. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr. 2009; 90(2):407–414. PMID:
19535427.

36. Kent PS, McCarthy MP, Burrowes JD, McCann L, Pavlinac J, Goeddeke-Merickel CM, et al. Academy of Nutrition and Dietetics and National Kidney Foundation: revised 2014 Standards of Practice and Standards of Professional Performance for registered dietitian nutritionists (competent, proficient, and expert) in nephrology nutrition. J Ren Nutr. 2014; 24(5):275–285.e45. PMID:
25167996.

37. Aparicio M. Protein intake and chronic kidney disease: literature review, 2003 to 2008. J Ren Nutr. 2009; 19(5):Suppl. S5–S8. PMID:
19712877.

38. Kalantar-Zadeh K, Moore LW, Tortorici AR, Chou JA, St-Jules DE, Aoun A, et al. North American experience with low protein diet for non-dialysis-dependent chronic kidney disease. BMC Nephrol. 2016; 17(1):90–100. PMID:
27435088.

39. Barril-Cuadrado G, Puchulu MB, Sánchez-Tomero JA. Table showing dietary phosphorus/protein ratio for the Spanish population. Usefulness in chronic kidney disease. Nefrologia. 2013; 33(3):362–371. PMID:
23640120.
40. Huang X, Lindholm B, Stenvinkel P, Carrero JJ. Dietary fat modification in patients with chronic kidney disease: n-3 fatty acids and beyond. J Nephrol. 2013; 26(6):960–974. PMID:
24249210.

41. Ewers B, Riserus U, Marckmann P. Effects of unsaturated fat dietary supplements on blood lipids, and on markers of malnutrition and inflammation in hemodialysis patients. J Ren Nutr. 2009; 19(5):401–411. PMID:
19541503.

42. Gopinath B, Harris DC, Flood VM, Burlutsky G, Mitchell P. Consumption of long-chain n-3 PUFA, α-linolenic acid and fish is associated with the prevalence of chronic kidney disease. Br J Nutr. 2011; 105(9):1361–1368. PMID:
21255476.

43. Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005; 28(1):164–176. PMID:
15616252.

44. Ellam T, Fotheringham J, Kawar B. Differential scaling of glomerular filtration rate and ingested metabolic burden: implications for gender differences in chronic kidney disease outcomes. Nephrol Dial Transplant. 2014; 29(6):1186–1194. PMID:
24235074.

45. Garofalo C, Borrelli S, Provenzano M, De Stefano T, Vita C, Chiodini P, et al. Dietary salt restriction in chronic kidney disease: a meta-analysis of randomized clinical trials. Nutrients. 2018; 10(6):732–746.

46. McMahon EJ, Campbell KL, Bauer JD, Mudge DW. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev. 2015; 18(2):CD010070.

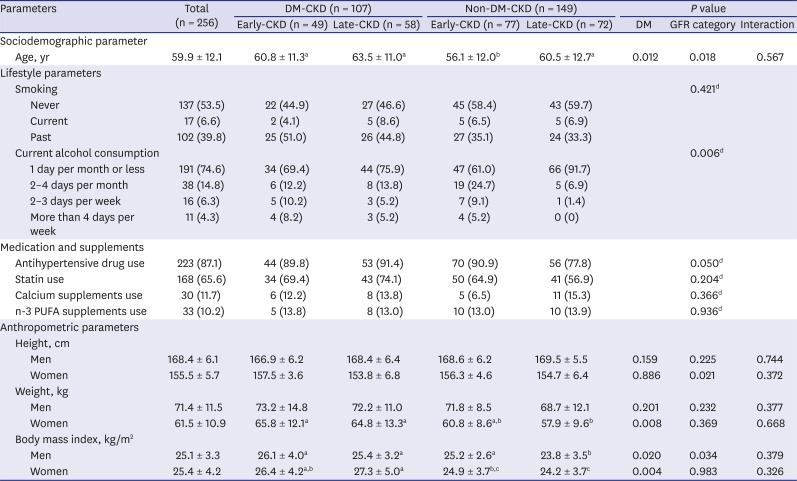
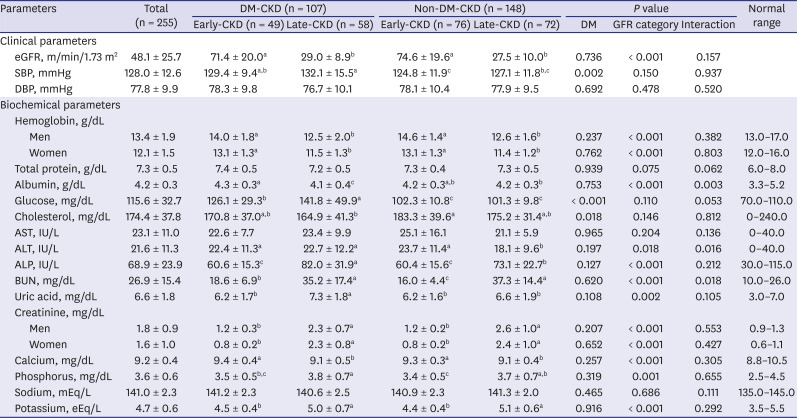
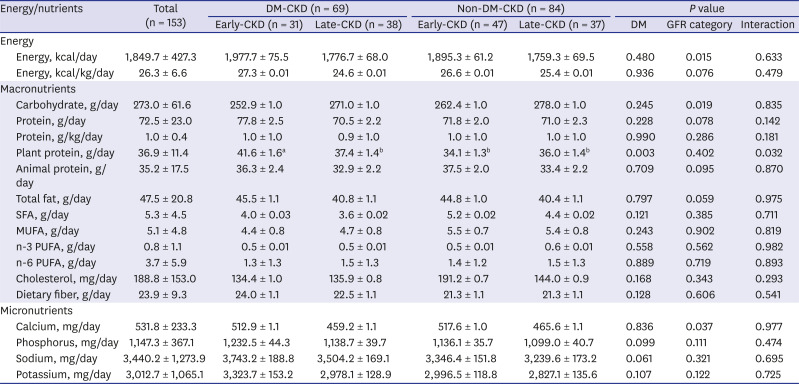
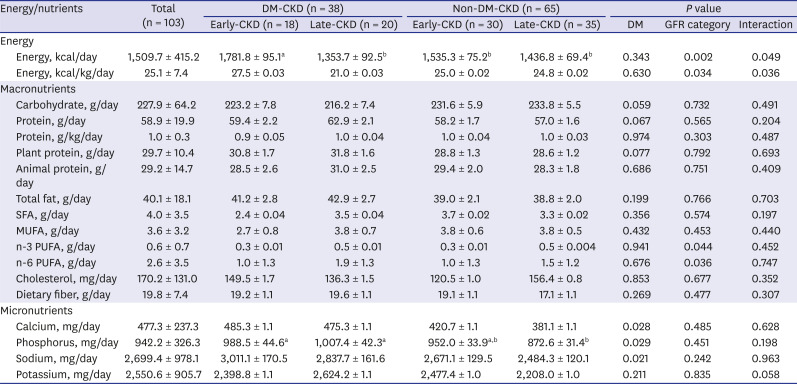




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download