The coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a pandemic infectious disease threatening the world. In Daegu, Korea, the outbreak started on February 18, 2020, and peaked on February 29, 2020, with 741 new cases confirmed in a day.
1 In Korea, the method used for the diagnosis of all cases of COVID-19 was real-time polymerase chain reaction.
With an explosive increase in the number of new patients, hospital bed shortage was a great challenge to the healthcare system.
1 We developed and employed a remote telephone severity scoring system (Daegu Severity Score for COVID-19) for assigning priority for hospitalization and arranging for facility isolation (“therapeutic living centers”) starting on February 29, 2020.
1 Fifteen centers were operated for the 3,033 admissions to COVID-19 therapeutic living centers.
1 Approximately 150 physicians of the Daegu Medical Association (DMA) voluntarily participated in this study and checked the status of patients who were staying at home on a daily basis.
1 They reported the interview results to the team arranging hospitalization or facility isolation in Daegu. During the interviews, several DMA physicians found that a significant number of the patients stated experiencing acute loss of smell (anosmia) or loss of taste (ageusia). Acute smell and taste disorders are related to a wide range of respiratory viral infections.
23
COVID-19 is characterized by a variety of clinical manifestations.
4 In a typical case, a high fever appears after dry cough; in some cases, viral pneumonia develops and progresses, resulting in shortness of breath.
45 Common symptoms among patients with COVID-19 include fever, dry cough, shortness of breath (dyspnea), muscle ache (myalgia), confusion, headache, sore throat, rhinorrhea, chest pain, diarrhea, nausea/vomiting, conjunctival congestion, nasal congestion, sputum production, fatigue (malaise), hemoptysis, and chills.
46789 A literature review revealed a few published articles on the importance of anosmia or ageusia as symptoms of COVID-19.
10111213
From March 8, 2020, DMA physicians prospectively questioned patients newly diagnosed with COVID-19 who were awaiting hospitalization or facility isolation regarding the presence of anosmia or ageusia; they also provided counseling on a daily basis for these symptoms until admission to hospitals or therapeutic living centers.
The data collected on anosmia or ageusia during the telephone severity scoring performed from March 8, 2020 to March 31, 2020, were analyzed retrospectively for the evaluation of the diagnostic significance of anosmia or ageusia in COVID-19. Additional telephone calls were made after admission to assess the duration of symptom persistence among those who reported that anosmia or ageusia persisted until hospitalization or facility isolation.
We analyzed the collected data using descriptive statistics and Kaplan-Meier analysis for the evaluation of factors associated with the recovery from anosmia or ageusia. Statistical analyses were performed using R statistics version 3.5.
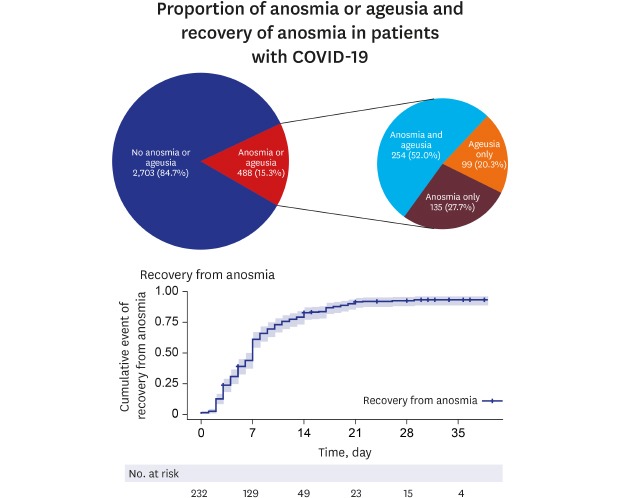
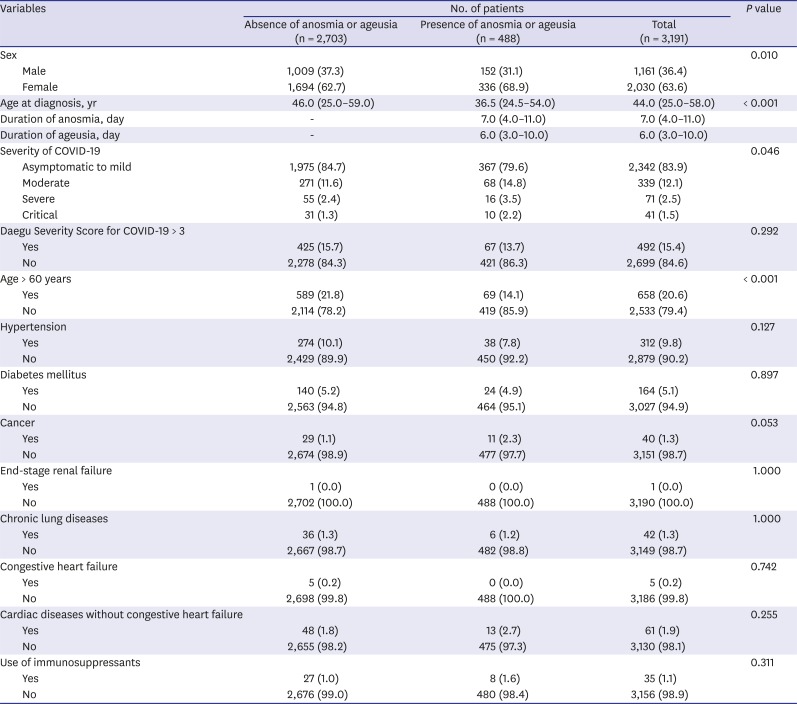
Approximately 15% (15.3%, 488/3,191) patients had anosmia or ageusia in the early stage of COVID-19 (
Fig. 1). Among patients with asymptomatic-to-mild disease severity (2,342 patients), 367 (15.7%) had anosmia or ageusia. The basic characteristics of the patients with or without anosmia or ageusia are summarized in
Table 1. Anosmia or ageusia was significantly more common among females and younger individuals (
P = 0.01 and
P < 0.001, respectively) (
Table 1).
Fig. 1
Proportion of anosmia or ageusia in patients with coronavirus disease 2019 confirmed by polymerase chain reaction.

Table 1
Basic characteristics of patients with confirmed COVID-19 with or without anosmia or ageusia
|
Variables |
No. of patients |
P value |
|
Absence of anosmia or ageusia (n = 2,703) |
Presence of anosmia or ageusia (n = 488) |
Total (n = 3,191) |
|
Sex |
|
|
|
0.010 |
|
Male |
1,009 (37.3) |
152 (31.1) |
1,161 (36.4) |
|
Female |
1,694 (62.7) |
336 (68.9) |
2,030 (63.6) |
|
Age at diagnosis, yr |
46.0 (25.0–59.0) |
36.5 (24.5–54.0) |
44.0 (25.0–58.0) |
< 0.001 |
|
Duration of anosmia, day |
- |
7.0 (4.0–11.0) |
7.0 (4.0–11.0) |
|
|
Duration of ageusia, day |
- |
6.0 (3.0–10.0) |
6.0 (3.0–10.0) |
|
|
Severity of COVID-19 |
|
|
|
0.046 |
|
Asymptomatic to mild |
1,975 (84.7) |
367 (79.6) |
2,342 (83.9) |
|
Moderate |
271 (11.6) |
68 (14.8) |
339 (12.1) |
|
Severe |
55 (2.4) |
16 (3.5) |
71 (2.5) |
|
Critical |
31 (1.3) |
10 (2.2) |
41 (1.5) |
|
Daegu Severity Score for COVID-19 > 3 |
|
|
|
0.292 |
|
Yes |
425 (15.7) |
67 (13.7) |
492 (15.4) |
|
No |
2,278 (84.3) |
421 (86.3) |
2,699 (84.6) |
|
Age > 60 years |
|
|
|
< 0.001 |
|
Yes |
589 (21.8) |
69 (14.1) |
658 (20.6) |
|
No |
2,114 (78.2) |
419 (85.9) |
2,533 (79.4) |
|
Hypertension |
|
|
|
0.127 |
|
Yes |
274 (10.1) |
38 (7.8) |
312 (9.8) |
|
No |
2,429 (89.9) |
450 (92.2) |
2,879 (90.2) |
|
Diabetes mellitus |
|
|
|
0.897 |
|
Yes |
140 (5.2) |
24 (4.9) |
164 (5.1) |
|
No |
2,563 (94.8) |
464 (95.1) |
3,027 (94.9) |
|
Cancer |
|
|
|
0.053 |
|
Yes |
29 (1.1) |
11 (2.3) |
40 (1.3) |
|
No |
2,674 (98.9) |
477 (97.7) |
3,151 (98.7) |
|
End-stage renal failure |
|
|
|
1.000 |
|
Yes |
1 (0.0) |
0 (0.0) |
1 (0.0) |
|
No |
2,702 (100.0) |
488 (100.0) |
3,190 (100.0) |
|
Chronic lung diseases |
|
|
|
1.000 |
|
Yes |
36 (1.3) |
6 (1.2) |
42 (1.3) |
|
No |
2,667 (98.7) |
482 (98.8) |
3,149 (98.7) |
|
Congestive heart failure |
|
|
|
0.742 |
|
Yes |
5 (0.2) |
0 (0.0) |
5 (0.2) |
|
No |
2,698 (99.8) |
488 (100.0) |
3,186 (99.8) |
|
Cardiac diseases without congestive heart failure |
|
|
|
0.255 |
|
Yes |
48 (1.8) |
13 (2.7) |
61 (1.9) |
|
No |
2,655 (98.2) |
475 (97.3) |
3,130 (98.1) |
|
Use of immunosuppressants |
|
|
|
0.311 |
|
Yes |
27 (1.0) |
8 (1.6) |
35 (1.1) |
|
No |
2,676 (99.0) |
480 (98.4) |
3,156 (98.9) |

The duration of these two symptoms was ascertained based on the daily interviews conducted by DMA physicians during the waiting period for hospitalization or facility isolation and by follow-up telephone interviews with 232 (for anosmia) and 196 (for ageusia) patients.
Recovery from anosmia is expressed using a survival curve (
Fig. 2A). Kaplan-Meier graphs with log-rank tests were generated using data on recovery from anomia based on demographic variables including age of > 50 years and sex. No significant differences were observed in log-rank tests.
Fig. 2
Graph of recovery from anosmia and ageusia among patients with coronavirus disease 2019. (A) Recovery from anosmia. (B) Recovery time pattern of anosmia. (C) Recovery time pattern of ageusia. (D) Pattern of persistence of anosmia according to age group.
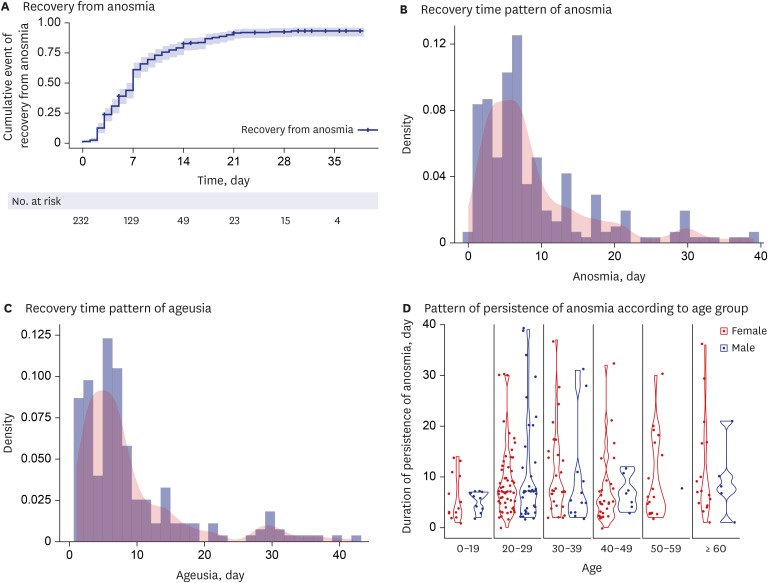

The median time to recovery from anosmia was 7 days, and the recovery time pattern is depicted in
Fig. 2B. The median time to recovery from ageusia was 7 days, and the recovery time pattern is shown in
Fig. 2C. Most patients with anosmia or ageusia recovered within 3 weeks (
Fig. 2B and C). Young age, particularly the age group of 20–39 years, showed a tendency to be associated with a longer persistence of anosmia (
Fig. 2D). Recovery from ageusia was similar to that from anomia (
Supplementary Fig. 1). Recently, anosmia was reported in a small cross-sectional survey study of COVID-19.
11 This article did not report follow-up information and included a relatively small number of patients (59 patients). Our data were derived from 3,191 patients, among whom 232 (anosmia) and 143 (ageusia) were followed up regarding the persistence of these symptoms.
Smell and taste disorders are related to a wide range of viral infections.
23 Infection of the upper respiratory tract can cause acute-onset anosmia or ageusia because of viral damage to the olfactory epithelium.
3 Moreover, viruses that can use the olfactory nerve as a route into the central nervous system include influenza A virus, herpesviruses, poliovirus, rabies virus, parainfluenza virus, adenoviruses, and Japanese encephalitis virus.
2 In mouse models, SARS-CoV demonstrated transneuronal penetration through the olfactory bulb and its infection resulted in the rapid, transneuronal spread of the virus to connected areas of the brain.
14 In COVID-19, headache may not only be a constitutional symptom but also be a symptom induced by invasion of the central nervous system. Human angiotensin-converting enzyme 2 is a functional receptor for SARS-CoV-2.
1012
Damage to the olfactory nerve during invasion and multiplication of SARS-CoV-2 may explain anosmia observed in the early stage of COVID-19. Therefore, anosmia or ageusia may be more frequently observed in the COVID-19 patients than other respiratory viral infections.
Ageusia may be a secondary result of olfactory dysfunction. However, the angiotensin-converting enzyme 2 receptor, which is the main host cell receptor of SARS-CoV-2 for binding and penetrating cells, is widely expressed on epithelial cells of the oral mucosa.
15 Damage of mucosal epithelial cells of the oral cavity may explain ageusia observed in the early stage of COVID-19. This evidence may explain the pathogenetic mechanism underlying anosmia and ageusia in COVID-19.
High transmissibility of COVID-19 before and immediately after symptom onset was reported with a recent epidemic study.
16 Early diagnosis is important for the control of COVID-19, recognition of early signs such as anosmia or ageusia might be very helpful for the diagnosis COVID-19 and isolation of the patients.
This telephone severity scoring system had a limitation regarding the accuracy of the assessment of patients. However, anosmia and ageusia are not ambiguous symptoms. Our report had a relatively large number of patients and focused on the time pattern on the recovery of these symptoms.
In conclusion, anosmia and ageusia seem to be part of important symptoms and clues for the diagnosis of COVID-19, particularly in the early stage of the disease. The acute anosmia or ageusia need to be recognized as important symptoms of the COVID-19 infection.
Among patients with asymptomatic-to-mild disease severity, the presence of anosmia or ageusia may be an important differential presentation for the suspicion and diagnosis of COVID-19. And these symptoms may recover within 3 weeks.