Globally, gastric cancer is the fifth most common cancer and the third most common cause of death from cancer. The incidence of gastric cancer is particularly high in East Asia, including Japan and the Korea.
1 Although the incidence of gastric cancer in Korea has decreased slightly since 2011, it is the most common cancer in men and the third most common cancer in women, except for thyroid cancer.
2 The high prevalence of
Helicobacter pylori infection in East Asia can only partially account for the high rate of gastric cancer
3 because the incidence is significantly higher in Eastern Asia than in regions with a similar
H. pylori infection prevalence.
Alcohol is associated with various cancers. In particular, the association between upper aerodigestive tract cancer and alcohol consumption is well-established.
4 Although several previous studies have shown an association between alcohol consumption and gastric cancer,
567 the relationship is not as strong as that reported for esophageal and oropharyngeal cancers (i.e., dose-response relationships). However, previous research has shown that the association between alcohol consumption and gastric cancer is stronger in East Asia than in other regions.
6
An aldehyde dehydrogenase 2 gene (
ALDH2) rs671 missense mutation that affects alcohol metabolism is common in East Asians.
8 Alcohol consumption causes an unpleasant alcohol-flush reaction in inactive
ALDH2 carriers; thus, these individuals tend to avoid drinking alcohol.
91011 However, findings regarding the association between rs671 variants and gastric cancer are conflicting
1213141516; therefore, we investigated the association between the
ALDH2 rs671 polymorphism and gastric cancer in a Korean population.
The case group consisted of 3,245 patients diagnosed with new-onset gastric cancer by biopsy at Chonnam National University Hwasun Hospital between April 2004 and February 2010. Patients under 50 years of age were excluded from the study because the control subjects were drawn from a community-based cohort study of adults aged 50 years and older. Gastric cancer was classified according to anatomical site as per the International Classification of Disease 10th edition (ICD-10) as cardia (C16.0), non-cardia (C16.1–16.8), and unspecified (C16.9) and histological type: intestinal, diffuse, mixed type, and unspecified.
The control group included participants from the Dong-gu study, a prospective cohort study of risk factors for chronic diseases including stroke, coronary heart disease, cognitive decline, cancer, and fracture, in Korean adults.
17 The Dong-gu study consisted of 9,260 participants aged 50 years and older. Of those, 455 participants with a history of cancer were excluded. Because of missing data, our analysis included 8,732 participants.
Genomic DNA was extracted from peripheral blood using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. The ALDH2 rs671 polymorphism was genotyped by high-resolution melting (HRM) analysis using a Rotor-Gene 6000TM (Corbett Research, Sydney, Australia). Polymerase chain reaction (PCR) primers producing a 97-bp amplicon were as follows: forward primer, 5′-TTGGTGGCTACAAGATGTCG-3′ and reverse primer, 5′-CAGGTCCCACACTCACAGTTT-3′. The reaction mixture for HRM included 200 nM PCR primer, 1 µM SYTO 9 fluorescent dye (Invitrogen, Carlsbad, CA, USA), 0.5U F-Star Taq DNA polymerase (BioFACT Co., Ltd., Daejeon, Korea), and 40 ng genomic DNA in 10 µL reaction volumes. The cycling conditions were started with denaturation at 95°C for 5 minutes, followed by 40 cycles of 95°C for 5 seconds and 58°C for 30 seconds.
Information on smoking history and alcohol consumption was obtained from the medical records of the case group and from interview questionnaires administered to the control group. Alcohol consumption was categorized as current drinker and abstainer in the medical records, and according to the amount and frequency of drinking in the Dong-gu study. Accordingly, we defined current drinkers as individuals who reported consuming one or more drinks per month and classified participants as current drinkers or abstainers. Smoking history was categorized as current smoker, ex-smoker, and never smoker in the medical records and the Dong-gu study. We classified participants as an ever-smokers (current or ex-smoker) or never smokers.
The baseline characteristics of the case and control participants were assessed according to gender. General characteristics were compared between groups using t-tests for continuous variables and χ2 tests for categorical variables. Multivariate logistic regression analysis was performed to assess the associations between the ALDH2 rs671 polymorphism and gastric cancer. Genotype was categorized as active ALDH2 (GG) and inactive ALDH2 (GA/AA). Active ALDH2 was used as a reference. All models were adjusted for age. Statistical significance was set at P values below 0.05. All analyses were performed using R (version 3.6.1; R Project, Vienna, Austria).
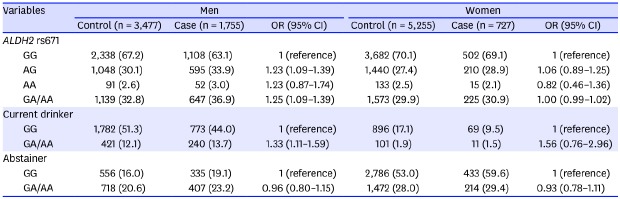
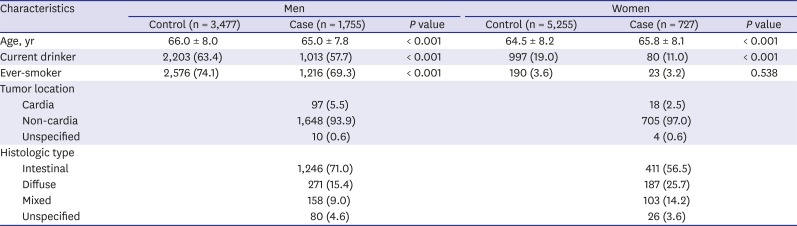
Table 1 shows the baseline characteristics of the case and control groups according to gender. Men in the control group were older than those in the case group, whereas the women in the case group were older than those in the control group. The current drinker rate was higher in the control group than in the case group in both men and women. The smoking rate was significantly higher in men in the control group than among those in the case group. The non-cardia site was the most common tumor location in men (94.0%) and women (97.0%). The most common histological type was intestinal, followed by the diffuse and the mixed types in both gender.
Table 1
Baseline characteristics of cases and controls according to gender
|
Characteristics |
Men |
Women |
|
Control (n = 3,477) |
Case (n = 1,755) |
P value |
Control (n = 5,255) |
Case (n = 727) |
P value |
|
Age, yr |
66.0 ± 8.0 |
65.0 ± 7.8 |
< 0.001 |
64.5 ± 8.2 |
65.8 ± 8.1 |
< 0.001 |
|
Current drinker |
2,203 (63.4) |
1,013 (57.7) |
< 0.001 |
997 (19.0) |
80 (11.0) |
< 0.001 |
|
Ever-smoker |
2,576 (74.1) |
1,216 (69.3) |
< 0.001 |
190 (3.6) |
23 (3.2) |
0.538 |
|
Tumor location |
|
|
|
|
|
|
|
Cardia |
|
97 (5.5) |
|
|
18 (2.5) |
|
|
Non-cardia |
|
1,648 (93.9) |
|
|
705 (97.0) |
|
|
Unspecified |
|
10 (0.6) |
|
|
4 (0.6) |
|
|
Histologic type |
|
|
|
|
|
|
|
Intestinal |
|
1,246 (71.0) |
|
|
411 (56.5) |
|
|
Diffuse |
|
271 (15.4) |
|
|
187 (25.7) |
|
|
Mixed |
|
158 (9.0) |
|
|
103 (14.2) |
|
|
Unspecified |
|
80 (4.6) |
|
|
26 (3.6) |
|

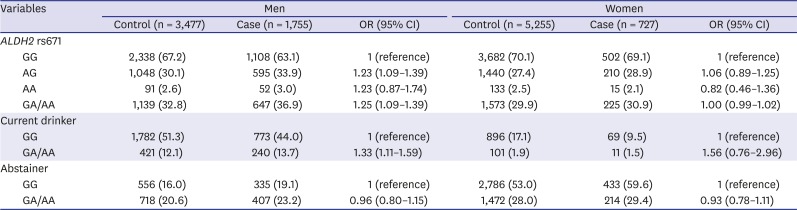
Table 2 shows the distribution of the
ALDH2 rs671 genotypes and the odds ratios (ORs) and 95% confidence intervals (CIs) for gastric cancer according to gender and alcohol consumption. Using active
ALDH2 as the reference group, inactive
ALDH2 was associated with an increased risk of gastric cancer (OR, 1.25; 95% CI, 1.09–1.39) in men, but not in women (OR, 1.00; 95% CI, 0.98–1.02). The association between ALDH2 rs671 polymorphism and gastric cancer was more evident in current drinkers than in abstainers. In men, compared to active
ALDH2 genotype, the ORs of inactive
ALDH2 were 1.33 (95% CI, 1.11–1.59) for current drinker and 0.96 (95% CI, 0.80–1.15) for abstainer (
P for interaction = 0.012). In women, the OR for current drinker was higher than that for abstainer; however, the difference was not statistically significant (
P for interaction = 0.197).
Table 2
Distribution of ALDH2 rs671 genotype and age-adjusted odd ratios for gastric cancer according to gender
|
Variables |
Men |
Women |
|
Control (n = 3,477) |
Case (n = 1,755) |
OR (95% CI) |
Control (n = 5,255) |
Case (n = 727) |
OR (95% CI) |
|
ALDH2 rs671 |
|
|
|
|
|
|
|
GG |
2,338 (67.2) |
1,108 (63.1) |
1 (reference) |
3,682 (70.1) |
502 (69.1) |
1 (reference) |
|
AG |
1,048 (30.1) |
595 (33.9) |
1.23 (1.09–1.39) |
1,440 (27.4) |
210 (28.9) |
1.06 (0.89–1.25) |
|
AA |
91 (2.6) |
52 (3.0) |
1.23 (0.87–1.74) |
133 (2.5) |
15 (2.1) |
0.82 (0.46–1.36) |
|
GA/AA |
1,139 (32.8) |
647 (36.9) |
1.25 (1.09–1.39) |
1,573 (29.9) |
225 (30.9) |
1.00 (0.99–1.02) |
|
Current drinker |
|
|
|
|
|
|
|
GG |
1,782 (51.3) |
773 (44.0) |
1 (reference) |
896 (17.1) |
69 (9.5) |
1 (reference) |
|
GA/AA |
421 (12.1) |
240 (13.7) |
1.33 (1.11–1.59) |
101 (1.9) |
11 (1.5) |
1.56 (0.76–2.96) |
|
Abstainer |
|
|
|
|
|
|
|
GG |
556 (16.0) |
335 (19.1) |
1 (reference) |
2,786 (53.0) |
433 (59.6) |
1 (reference) |
|
GA/AA |
718 (20.6) |
407 (23.2) |
0.96 (0.80–1.15) |
1,472 (28.0) |
214 (29.4) |
0.93 (0.78–1.11) |

We investigated the association between
ALDH2 rs671 polymorphism and gastric cancer. Inactive
ALDH2 was associated with an increased risk of gastric cancer in men, but not in women. Moreover, this association was stronger in current drinkers than in abstainers. Our findings are consistent with those of Yang et al.
16 who found a significant association between the
ALDH2 genotype and gastric cancer in men only. These findings may be explained by the different drinking behaviors in men and women: given the higher proportion of current drinkers, the risks presented by the inactive
ALDH2 genotype is greater for men than for women. In contrast, Zhang et al.
15 found no association between
ALDH2 and gastric cancer in men or women. We found that the association between
ALDH2 and gastric cancer was modified by alcohol consumption. Similarly, several previous studies found a significant association between the
ALDH2 genotype and gastric cancer only in individuals with higher alcohol consumption.
121314 Conversely, a case-control study conducted by the National Cancer Center Korea found an association between inactive
ALDH2 and higher risk of gastric cancer, regardless of the amount of alcohol consumed.
16 This discrepancy may be explained by differences in alcoholic beverage preferences, polymorphism distribution across populations, sample size, or the classification of alcohol consumption.
The reason of increased gastric cancer risk in inactive
ALDH2 carriers may be due to the carcinogenic effect of aldehyde accumulation. Acetaldehyde associated with alcoholic beverages is classified as a group 1 carcinogen by the International Agency for Research on Cancer.
18 Previous studies have shown that after consuming ethanol, the acetaldehyde concentration in the gastric juice of inactive
ALDH2 carriers was 5.6-fold higher than that of active
ALDH2 carriers.
19
The major strength of our study is the large sample size; ours is the largest case-control investigation of the association between the
ALDH2 rs671 polymorphism and gastric cancer. However, our study has several limitations. First, we did not investigate the effect of another polymorphism such as alcohol dehydrogenase 1B (
ADH1B) rs1229984 that are involved in alcohol metabolism. However, previous studies
1214 found no association between
ADH1B and the risk of gastric cancer and no evidence of gene-to-gene interactions between
ADH1B and
ALDH2. Second, we did not investigate a dose-response relationship between alcohol consumption and gastric cancer.
In conclusion, our case-control study found an association between ALDH2 rs671 polymorphism and gastric cancer in a Korean population, suggesting that the carcinogenic effect of alcohol may differ according to the ALDH2 genotype. Therefore, preventative strategies for alcohol-related cancer may need to differ according to the ALDH2 genotype in East Asian populations.
Ethics Statement
At the time of their peripheral blood collections, all case and control subjects provided their informed consent to participate in this study. This study was reviewed and approved by the Institutional Review Boards (IRBs) of Chonnam National University Hospital Hwasun (CUNH IRB-2014-016) and Chonnam National University Hospital (I-2008-05-056).
Go to :