INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease characterized by a wide spectrum of clinical manifestations affecting various organs and tissues and heterogeneous pathogenic mechanisms.
12 The mortality rate of patients with lupus is estimated to be about three fold higher than that of the age- and gender-matched general population.
3 Cardiovascular disease (CVD) is one of the leading causes of increasing morbidity and mortality in patients with SLE, together with infection and adverse effects due to therapeutic strategies.
4567 Earlier studies observed that the prevalence of clinical cardiac diseases in SLE ranged from 8% to 10% according to study population and CVD outcomes.
89 In particular, epidemiological evidence suggests that patients with SLE are about 9 to 50 fold more likely to experience increased risk of CVD including myocardial infarction and angina pectoris, compared to the general population.
1011
The risk of development of CVD in SLE is related not only to traditional risk factors such as older age, hypertension, dyslipidemia, and diabetes mellitus, but also to disease-specific factors such as disease activity, autoantibodies, major organ damage, and treatment modality.
1012 However, traditional risk factors, such as those proposed in the general population, do not fully explain the outcomes of CVD in SLE. Furthermore, the relationships between disease-specific factors and CVD outcomes vary depending on the characteristics of the study population, including population size, origin and ethnicity. One epidemiologic study conducted using the Korean National Health Insurance Service database revealed that SLE itself was an independent risk factor for CVD in Korean population.
13 However, few data about traditional and disease-related risk factors for cardiac involvements including vascular and non-vascular events in Korean patients with SLE are available. Therefore, the aim of this study was to investigate traditional and lupus-related risk factors of cardiac involvement in patients with SLE recruited from a nationwide prospective multicenter-based registry.
Go to :

METHODS
Study population
Six university-based medical centers participated in the Korean Lupus Network (KORNET) registry, which was a prospective study, from January 2014 to December 2018. All subjects were recruited by rheumatologists at the outpatient clinics of each center and fulfilled the 1982 revised and 1997 updated American College of Rheumatology classification criteria for SLE.
1415 Four hundred thirty-seven patients who did not have any cardiac involvement were eligible for inclusion in this study.
Collection of clinical information
This study used clinical data collected from baseline to the latest follow-up. Clinical information at baseline included age (years), gender, disease duration (months), smoking status (non-current smoker and current smoker), alcohol intake (current alcoholic and non-current alcoholic), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), and body mass index (BMI, kg/m
2). BMI was classified into two groups: normal (< 23.0) and overweight and obese (≥ 23.0) according to criteria proposed by the International Obesity Task Force for East Asian adults.
16
Comorbidities were identified through reviews of medical records and interviews with each subject and included hypertension, hyperlipidemia, diabetes mellitus, and renal disorders. Hypertension is defined when the condition was previously diagnosed and the patient is currently taking anti-hypertensive medications or has blood pressure 140/90 mmHg or above at the time of enrollment. Patients taking anti-hyperlipidemic drugs due to hypercholesterolemia or hypertriglyceridemia or with fasting total cholesterol above 240 mg/dL, low-density lipoprotein cholesterol above 130 mg/dL, or triglycerides above 150 mg/dL were considered to have hyperlipemia. Diabetes mellitus is defined when previously diagnosed and the patient is currently taking anti-diabetic medications.
Assessments of SLE-related measures
Inflammatory and immunologic markers at baseline included erythrocyte sedimentation rate (mm/hr), C-reactive protein (mg/dL), complement 3 (mg/mL), complement 4 (mg/mL), and positivity for autoantibodies such as anti-dsDNA antibody, anti-Sm antibody, anti-Ro antibody, anti-La antibody, anti-RNP antibody, lupus anticoagulant, anti-cardiolipin antibody, and anti-β2GPI antibody. Disease activity was measured using the safety of estrogen in lupus erythematosus national assessment (SELENA)-SLE disease activity index (SLEDAI). Assessments for SLE-related organ damage were performed using the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index (SDI). Patients diagnosed with lupus nephritis or with abnormality in the urinalysis such as proteinuria and hematuria provided by the definition of SELENA-SLEDAI were regarded as having renal involvement.
Determination of cardiac involvement in SLE
New onset cardiac events were identified using data from baseline to the latest follow-up. In this study we identified vascular cardiac events such as angina pectoris and myocardial infarction, and other non-vascular cardiac involvement including cardiomyopathy, valvular heart disease, and pericarditis. At the time of registry enrollment, patients without a history of cardiac diseases as described above were included. The onset of vascular or heart events such as stroke, congestive heart failure, peripheral vascular disease, and venous thrombus were excluded during the follow-up period.
Statistical analysis
Data were presented as median (interquartile range [IQR]) for quantitative (numeric) variables and numbers (%) for qualitative (categorical) variables. Kolmogorov-Smirnov analysis revealed that the data did not show a normal distribution. Therefore, non-parametric methods were used for statistical analysis. Comparisons of clinical variables between patients with and without cardiac manifestations were performed by χ2 tests, t-tests, or Fisher's exact tests for qualitative variables and Mann-Whitney U tests for quantitative variables, if available.
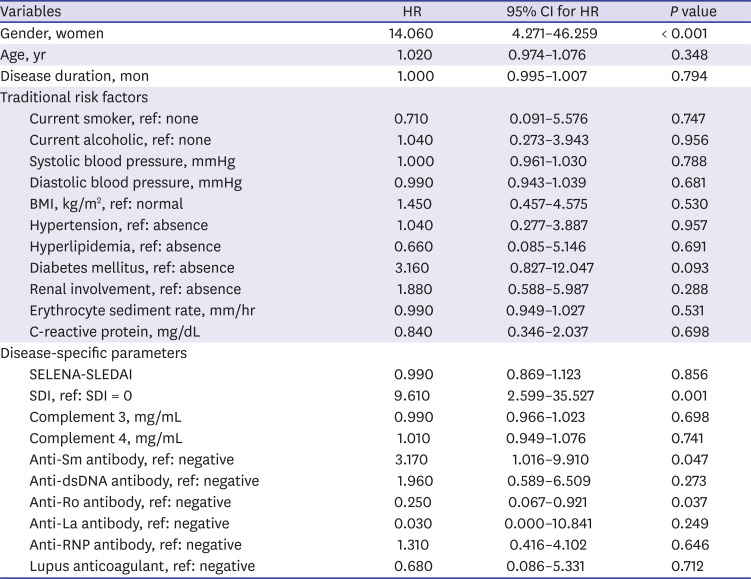
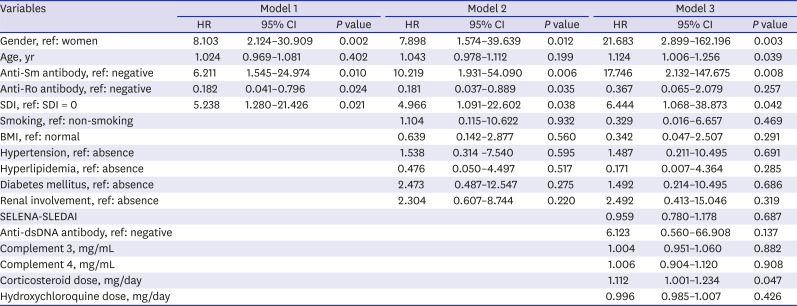
We used the Cox proportional hazard regression model to determine whether variables at baseline influence the development of CVD. In model 1, variables that differed significantly between patients with and without CVD were used as independent variables for crude analysis. Model 2 and model 3 were calculated after adjusting for additional confounding variables such as traditional risk factors, disease-specific activity indexes, and medications for adjusted analysis. In model 2, traditional risk factors such as smoking, BMI, hypertension and hyperlipidemia, diabetes mellitus and renal involvement were added to model 1. In model 3, disease-specific activity indexes and medications such as SELENA-SLEDAI, anti-dsDNA antibody, complement 3, complement 4, corticosteroid dose, hydroxychloroquine dose were added to model 2.
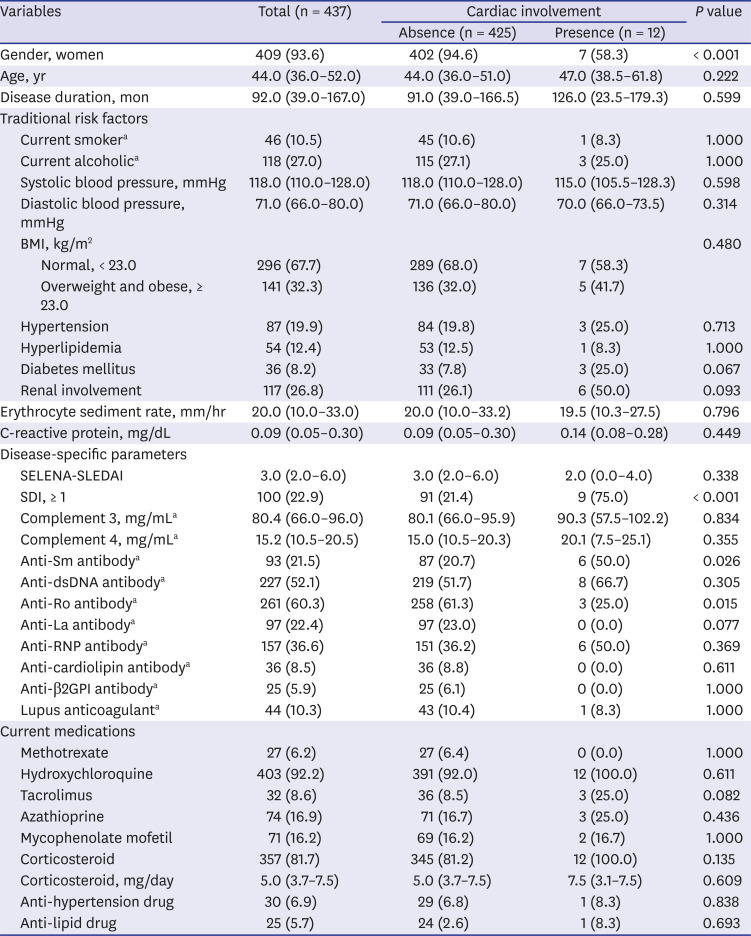
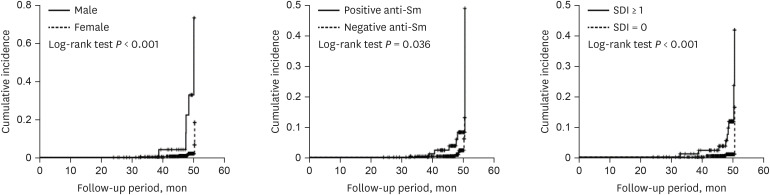
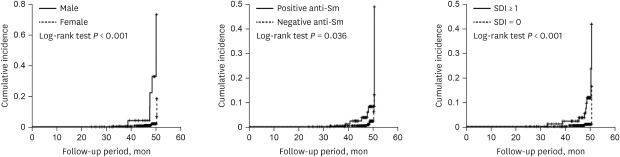
Cumulative incidence rates of CVD by gender, anti-Sm antibody, and SDI were estimated by the Kaplan-Meier method and the log-rank tests were used to compare between two incidence curves. The proportional hazards assumption was checked by log minus log plot. The curves by group variables were not cross and maintain parallel. Therefore, the ratio of the hazards for any two individuals is constant over time. The data were described as hazard ratio (HR) with 95% confidence interval (CI) for CVD. Cumulative incidence rates of CVD using candidates such as gender, anti-Sm antibody, and SDI were estimated by the Kaplan-Meier method. The data were described as incidence rate with 95% CI for each candidate. P values < 0.05 were considered to indicate statistical significance. The statistical analyses were performed using IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA).
Ethics statement
At the time of enrollment in the KORNET registry, all subjects gave written informed consent for inclusion in this study. This study was approved by the Institutional Review Board of Daegu Catholic University Medical Center (CR-19-097). This study was performed according to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Go to :

DISCUSSION
It is recognized that patients with SLE have lower life expectancies than the general population.
1718 Infection, malignancy, renal diseases, and CVD are considered major causes of mortality in SLE patients. Over decades, several studies have focused on causes or risk factors of CVD in SLE. In addition to traditional risk factors identified in the general population, several lupus-specific factors were found to be associated with the onset of CVD in patients with SLE.
1012 However, the results of these studies were somewhat heterogeneous depending on the characteristics of the study population. Here, we determined whether traditional and disease-specific risk factors are related to the development of vascular and non-vascular cardiac involvement including angina pectoris, myocardial infarction, cardiomyopathy, valvular heart disease, and pericarditis over a median follow-up period of about 47.6 months. We observed that factors such as men, older age, positive anti-Sm antibody, SDI, and corticosteroid use at baseline were associated with increased risk of cardiac involvement in SLE.
Increasing evidence indicates that lupus-related autoantibodies might be involved in atherosclerosis in cardiovascular events, including ischemic heart disease and stroke in SLE. There are few studies of the relationships between cardiac involvement and lupus-specific antibodies. Considering the role of anti-dsDNA antibody as a lupus-specific biomarker related to disease activity, its positivity was significantly associated with premature atherosclerosis manifesting as coronary plaque in patients with SLE (
P = 0.032).
19 In contrast, we did not detect an association between the presence of anti-dsDNA antibody at baseline and development of cardiac involvement (
P = 0.137). In general, there is evidence that anti-phospholipid antibodies contribute to endothelial dysfunction through induction of vascular inflammation and apoptosis and are associated with the development of CVD in SLE. The LUMINA cohort study found that any antiphospholipid antibody at baseline predicted occurrence of vascular events during a median follow-up duration of 73.8 months (HR, 3.463,
P = 0.007).
20 Similarly, Gustafsson et al.
21 demonstrated an independent predictor of antiphospholipid antibodies including anti-cardiolipin antibody IgG and anti-β2GP1 IgG for cardiovascular events (HR, 2.57,
P = 0.02; HR, 2.57,
P = 0.02, respectively). In contrast, there were no differences of non-vascular cardiac involvement including left ventricular mass, left ventricular hypertrophy or ejection fraction between patients with and without antiphospholipid antibody.
22 In our study, we failed to demonstrate significant differences in frequencies of anti-phospholipid antibodies between patients with and without cardiac involvement. In other words, we observed that patients with anti-Sm antibody were at increased risk of CVD compared to those without anti-Sm antibody (HR, 17.746;
P = 0.008). However, most studies found no association between anti-Sm antibody and the risk of CVD in SLE.
2123 Although the roles of autoantibodies in previous studies were lack of consistency, it suggests that some lupus-related autoantibodies play crucial roles in the development of cardiac involvement through activation of inflammation and autoimmunity. Further research on this relationship between anti-Sm antibody and cardiac involvement needs to be verified.
It has been recognized that the risk of developing cardiac involvement may increase if tissue or organ damage in patients with SLE is present during disease course. SDI, a representative tool that measures cumulative damage during the course of SLE, was found to be predictive of deleterious outcomes such as hospitalization and death in SLE patients.
24 In an analysis of 442 SLE patients in the LUMINA cohort, SDI was associated with vascular arterial events including angina pectoris, myocardial infarction, and stroke (odds ratio [OR], 1.271, 95% CI, 1.134–1.425,
P < 0.001).
25 In a multicenter retrospective case-control study, patients with at least one SDI tended to have CVD in univariate analysis, although statistical significance was lost after adjustments for age and gender.
23 However, hazard model analysis in our study revealed that the presence of at least one damage score (SDI ≥ 1) increased risk of cardiac involvement after adjustments for confounding factors (HR, 6.444,
P = 0.042). It will be necessary to confirm whether lupus-related tissue or organ damage predicts the risk of cardiac involvement in a larger study population.
Consistent with findings in the general population, traditional risk factors such as obesity, older age, hypertension, dyslipidemia, and diabetes mellitus remain crucial to the development of CVD in SLE.
1012 Considering the effect of gender on cardiovascular involvement, evidence that men are at elevated risk for the development of CVD has previously been addressed. In an analysis of 1979 patients in the Hopkins Lupus Cohort, men patients had more frequent CVD such as angina, myocardial infarction, and left ventricular hypertrophy than women based on SDI.
26 Stefanidou et al.
27 reported that men had higher prevalence of vascular thrombosis and stroke compared to women SLE patients (OR, 5.832,
P < 0.001; OR = 12.289,
P < 0.001, respectively). The GLADEL cohort showed that there were no differences in cardiovascular events between men and women SLE patients at the onset of disease, but cumulative incidence increased in men during the course of disease.
28 Consistently, we also found that men lupus patients had greater than 18-fold increased risk of cardiac involvement. However, a systemic review and meta-analysis of a total 11,934 SLE patients across 16 studies recently demonstrated that there were no significant differences of CVD and pericarditis between men and women (OR,1.43, 95% CI, 0.93–2.19; OR, 1.19, 95% CI, 0.97–1.45).
29 Cardiovascular events including myocardial infarction and valvular heart disease did not differ between genders based on SDI in a retrospective analysis of Korean SLE patients at a single tertiary hospital.
30 In another aspect, older age has consistently been regarded as a potent risk factor for CVD. Gustafsson et al.
21 demonstrated that age at baseline predicted approximately 2.4-fold increased risk of cardiovascular events such as ischemic heart disease, cerebrovascular or peripheral vascular disease and death. Another study revealed that age at study entry was significantly associated with cardiovascular events in SLE.
5 We also confirmed that age at baseline was an independent risk factor for cardiac involvement. Whether traditional risk factors such as gender and age have unfavorable effects on cardiac involvement should be identified in further studies.
There is evidence that therapeutic medications for SLE may be independent risk factors for the development of CVD. Corticosteroids are known to have beneficial effects against SLE by blocking inflammation and reducing organ damage.
12 This can be an expected preventive effect of corticosteroid on the occurrence of CVD. In contrast, high dose and long-term steroid use were found to increase risk of CVD through the induction of hypertension, hyperlipidemia, and insulin resistance.
1012 In this study, we found that corticosteroid use increased the risk of cardiac involvement after adjusting for confounding factors in Cox proportional hazard model analysis (HR, 1.112,
P = 0.047). Hydroxychloroquine, commonly used in SLE, is protective against risk of cardiovascular events including myocardial infarction, angina, and stroke through favorable effects on lipid profiles and glucose control.
31 However, in our study we did not observe lower risk of cardiac involvement in patients who used hydroxychloroquine compared to those who did not, similar to other earlier studies.
2123 There is still a debate about the effect of medications on cardiovascular events, further consideration is needed through future prospective studies.
There are some limitations in understanding the results of this study. First, this study was to identify new development of vascular and non-vascular cardiac events during a mean follow-up of 47.6 months or more in SLE patients who had no cardiac diseases at the beginning of enrollment. Cumulative incidence of cardiac diseases was only 2.7%, which is likely to be lower than the rate shown in previous cohort study for patients with cutaneous lupus erythematosus.
32 In Korean lupus patients for six-year follow-up, the incidence rates of myocardial infarction and heart failure were about 1.76 and 3.11, respectively.
13 It is considered to be similar to or slightly higher than our results, and is partly consistent with this study. Second, the number of patients enrolled in this study seems to be too small to fully reflect our finding in actual clinical practice. However, the data collection and management of our study are relatively well-designed. Therefore, it can overcome this weakness to some extent.
In conclusion, the novel finding of this study is that disease-specific factors such as anti-Sm antibody, SDI, and corticosteroid use are associated with increased risk of vascular and non-vascular cardiac involvement including angina pectoris, myocardial infarction, cardiomyopathy, valvular heart disease, and pericarditis in Korean patients with SLE, in addition to traditional factors including men gender and older age. Earlier studies reported that disease activity indexes such as SLEDAI or Systemic Lupus Activity Measure predict the risk of developing CVD.
525 However, we failed to identify a predictive role for disease activity indexes such as SELENA-SLEDAI and complements for the risk of the development of cardiac involvement. The results of this study provide a clinically meaningful evidence for the prevention and management of cardiac involvement in patients with SLE. In order to prevent and manage cardiac diseases in patients with SLE, physical examination, laboratory tests on autoantibodies, and presence of tissues and organ damage need to be carried out periodically, and if possible, efforts to reduce the use of steroids are considered.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download