1. Myerberg RJ, Castellanos A. Cardiac arrest and sudden cardiac death. In : Bonow RO, Mann DL, Zipes DP, Libby P, Braunwald E, editors. Braunwald's Heart Disease. 9th ed. Philadelphia, PA: Elsevier Saunders;2012. p. 845–884.
2. Waldmann V, Bougouin W, Karam N, Dumas F, Sharifzadehgan A, Gandjbakhch E, et al. Characteristics and clinical assessment of unexplained sudden cardiac arrest in the real-world setting: focus on idiopathic ventricular fibrillation. Eur Heart J. 2018; 39(21):1981–1987. PMID:
29566157.

3. Tateishi K, Abe D, Iwama T, Hamabe Y, Aonuma K, Sato A. Clinical value of ST-segment change after return of spontaneous cardiac arrest and emergent coronary angiography in patients with out-of-hospital cardiac arrest: Diagnostic and therapeutic importance of vasospastic angina. Eur Heart J Acute Cardiovasc Care. 2018; 7(5):405–413. PMID:
28730843.

4. Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, et al. The who, what, why, when, how and where of vasospastic angina. Circ J. 2016; 80(2):289–298. PMID:
26686994.

5. Waldmann V, Bougouin W, Karam N, Narayanan K, Sharifzadehgan A, Spaulding C, et al. Coronary vasospasm-related sudden cardiac arrest in the community. J Am Coll Cardiol. 2018; 72(7):814–815. PMID:
30092958.

6. Beltrame JF, Sasayama S, Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol. 1999; 33(6):1442–1452. PMID:
10334407.

7. El Menyar AA. Drug-induced myocardial infarction secondary to coronary artery spasm in teenagers and young adults. J Postgrad Med. 2006; 52(1):51–56. PMID:
16534169.
8. Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses - a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012; 61(1):10–13. PMID:
22237030.
9. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med. 2014; 370(22):2063–2066. PMID:
24758595.
10. Cannon CP, Braunwald E. Unstable angina and non-ST elevation myocardial infarction. In : Bonow RO, Mann DL, Zipes DP, Libby P, Braunwald E, editors. Braunwald's Heart Disease. 9th ed. Philadelphia, PA: Elsevier Saunders;2012. p. 1178–1209.
11. Matsue Y, Suzuki M, Nishizaki M, Hojo R, Hashimoto Y, Sakurada H. Clinical implications of an implantable cardioverter-defibrillator in patients with vasospastic angina and lethal ventricular arrhythmia. J Am Coll Cardiol. 2012; 60(10):908–913. PMID:
22840527.

12. Da Costa A, Isaaz K, Faure E, Mourot S, Cerisier A, Lamaud M. Clinical characteristics, aetiological factors and long-term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3-year follow-up study of 91 patients. Eur Heart J. 2001; 22(16):1459–1465. PMID:
11482919.

13. Ahn JM, Lee KH, Yoo SY, Cho YR, Suh J, Shin ES, et al. Prognosis of variant angina manifesting as aborted sudden cardiac death. J Am Coll Cardiol. 2016; 68(2):137–145. PMID:
27386766.
14. Takagi Y, Yasuda S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, et al. Clinical characteristics and long-term prognosis of vasospastic angina patients who survived out-of-hospital cardiac arrest: multicenter registry study of the Japanese Coronary Spasm Association. Circ Arrhythm Electrophysiol. 2011; 4(3):295–302. PMID:
21406685.
15. Kim YJ, Min SY, Lee DH, Lee BK, Jeung KW, Lee HJ, et al. The role of post-resuscitation electrocardiogram in patients with ST-segment changes in the immediate post-cardiac arrest period. JACC Cardiovasc Interv. 2017; 10(5):451–459. PMID:
28279312.
16. Group JC; JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014; 78(11):2779–2801. PMID:
25273915.

17. Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004; 291(7):870–879. PMID:
14970067.
18. Meune C, Joly LM, Chiche JD, Charpentier J, Leenhardt A, Rozenberg A, et al. Diagnosis and management of out-of-hospital cardiac arrest secondary to coronary artery spasm. Resuscitation. 2003; 58(2):145–152. PMID:
12909376.

19. Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med. 2007; 262(2):173–183. PMID:
17645585.

20. Kugiyama K, Yasue H, Okumura K, Ogawa H, Fujimoto K, Nakao K, et al. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996; 94(3):266–271. PMID:
8759065.

21. Li YJ, Hyun MH, Rha SW, Chen KY, Jin Z, Dang Q, et al. Diabetes mellitus is not a risk factor for coronary artery spasm as assessed by an intracoronary acetylcholine provocation test: angiographic and clinical characteristics of 986 patients. J Invasive Cardiol. 2014; 26(6):234–239. PMID:
24907077.
22. Ro YS, Shin SD, Song KJ, Kim JY, Lee EJ, Lee YJ, et al. Risk of diabetes mellitus on incidence of out-of-hospital cardiac arrests: a case-control study. PLoS One. 2016; 11(4):e0154245. PMID:
27105059.

23. Ro YS, Shin SD, Song KJ, Lee EJ, Lee YJ, Kim JY, et al. Interaction effects between hypothermia and diabetes mellitus on survival outcomes after out-of-hospital cardiac arrest. Resuscitation. 2015; 90:35–41. PMID:
25725296.

24. Hirlekar G, Karlsson T, Aune S, Ravn-Fischer A, Albertsson P, Herlitz J, et al. Survival and neurological outcome in the elderly after in-hospital cardiac arrest. Resuscitation. 2017; 118:101–106. PMID:
28736324.

25. Lee BK, Lee SJ, Park CH, Jeung KW, Jung YH, Lee DH, et al. Relationship between age and outcomes of comatose cardiac arrest survivors in a setting without withdrawal of life support. Resuscitation. 2017; 115:75–81. PMID:
28392372.

26. Kim WY, Giberson TA, Uber A, Berg K, Cocchi MN, Donnino MW. Neurologic outcome in comatose patients resuscitated from out-of-hospital cardiac arrest with prolonged downtime and treated with therapeutic hypothermia. Resuscitation. 2014; 85(8):1042–1046. PMID:
24746783.

27. Mader TJ, Nathanson BH, Millay S, Coute RA, Clapp M, McNally B, et al. Out-of-hospital cardiac arrest outcomes stratified by rhythm analysis. Resuscitation. 2012; 83(11):1358–1362. PMID:
22490674.

28. Myerburg RJ, Kessler KM, Mallon SM, Cox MM, deMarchena E, Interian A Jr, et al. Life-threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary-artery spasm. N Engl J Med. 1992; 326(22):1451–1455. PMID:
1574091.

29. Kim YT, Shin SD, Hong SO, Ahn KO, Ro YS, Song KJ, et al. Effect of national implementation of utstein recommendation from the global resuscitation alliance on ten steps to improve outcomes from Out-of-Hospital cardiac arrest: a ten-year observational study in Korea. BMJ Open. 2017; 7(8):e016925.

30. Yasue H, Takizawa A, Nagao M, Nishida S, Horie M, Kubota J, et al. Long-term prognosis for patients with variant angina and influential factors. Circulation. 1988; 78(1):1–9. PMID:
3260150.

31. Bro-Jeppesen J, Annborn M, Hassager C, Wise MP, Pelosi P, Nielsen N, et al. Hemodynamics and vasopressor support during targeted temperature management at 33°C Versus 36°C after out-of-hospital cardiac arrest: a post hoc study of the target temperature management trial. Crit Care Med. 2015; 43(2):318–327. PMID:
25365723.
32. Bruschke AV, Proudfit WL, Sones FM Jr. Progress study of 590 consecutive nonsurgical cases of coronary disease followed 5-9 years. I. Arterographic correlations. Circulation. 1973; 47(6):1147–1153. PMID:
4709534.
33. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009; 360(3):213–224. PMID:
19144937.

34. Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging. 2009; 2(8):1009–1023. PMID:
19679290.

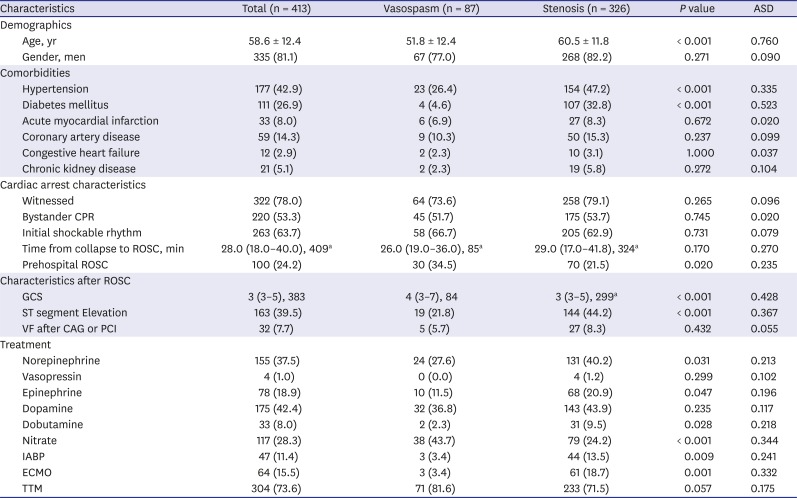
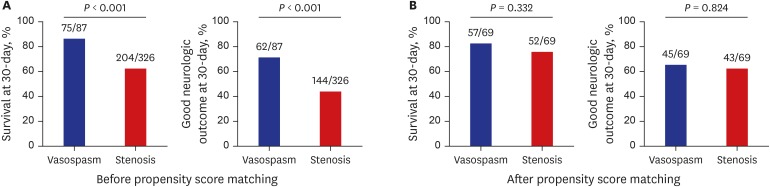
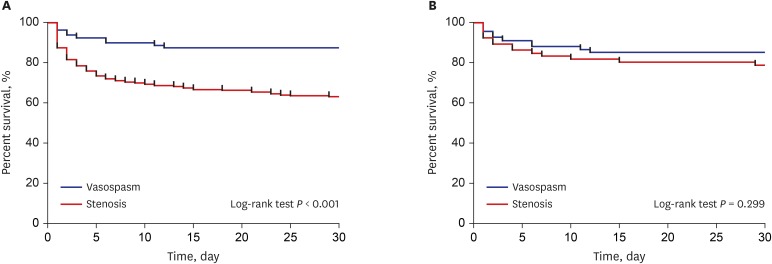
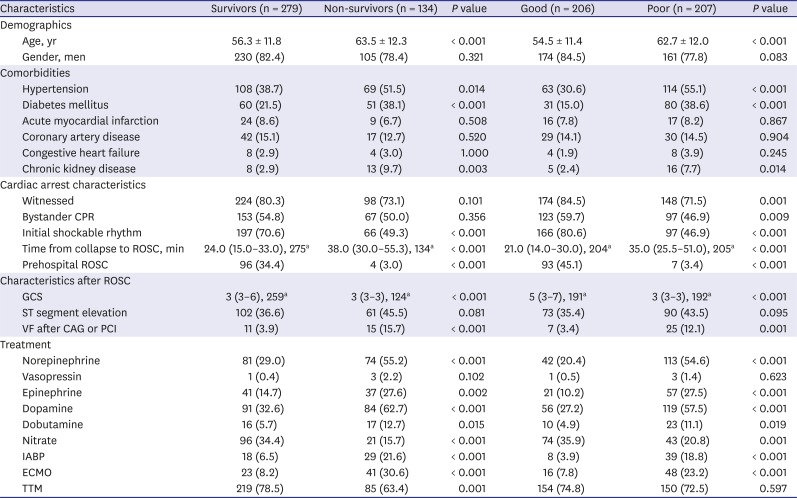




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download