Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are rare but extremely severe diseases affecting the skin and mucous membranes, with high mortality rates and the potential for permanent sequelae.
1 SJS/TEN symptoms include blisters, skin detachment, ocular surface epithelial defects, and/or pseudomembrane formation.
23 Such blisters and erosions cover 3%–10% of the body in patients with SJS, 11%–30% of the body in patients with overlapping SJS/TEN, and over 30% of the body in TEN patients.
45
SJS/TEN are caused by unpredictable off-target drug activity and comprise different types of idiopathic adverse drug reactions. The occurrence of SJS/TEN is influenced by genetic predisposition, various immunological mechanisms, environmental factors, and other causes.
167 Consequently, there are large variations in incidence rates among races.
1 Due to the rareness and ethnic specificity of these diseases, and differences in the pathways affected by the potential causative drugs, it is challenging to undertake large-scale and reproducible studies. Thus, the causes of SJS/TEN have not yet been fully delineated.
1567
There are several controversial hypotheses concerning the pathogenesis of SJS/TEN, including: 1) the Fas-associated death domain and caspase cascade pathway, 2) activation of perforin/granzyme B, 3) increase and activation of granulysin, and 4) pathways related to several cytokines/chemokines such as tumor necrosis factor-α.
12 However, the mechanisms associated with skin pathology and mucous membranes, particularly in the absence of dysfunction in other organs, have not been fully determined.
8
Although SJS/TEN is a very severe cutaneous adverse reaction, few effective treatment strategies are available other than corticosteroids and supportive care. Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into diverse cell types and are effective in tissue repair, tissue regeneration, and immunomodulation.
9 The purpose of the present study was to verify whether MSCs could be applied for the treatment of SJS/TEN. Therefore, we developed an SJS/TEN mouse model and investigated the therapeutic effects of MSCs on this model.
Blood sample was collected from a woman patient (45 years of age) with lamotrigine-induced SJS. Lamotrigine is a drug that can trigger severe cutaneous reactions, such as skin bullous changes and exfoliation, oral mucositis, and conjunctivitis. Peripheral blood mononuclear cells (PBMCs, 1.5 × 106 cells/mouse), containing drug-specific lymphocytes activated by lamotrigine, were isolated from the blood using LymphoprepTM (Axis Shield Diagnostics, Dundee, Scotland).
To generation of the SJS murine model, immunocompromised NOD/Shi-scid IL-2Rγ
null (NOG) mice (male, 6 weeks of age) were purchased from the Central Institute for Experimental Animals (Tokyo, Japan) and maintained under specific pathogen-free conditions.
8 We compared groups of mice based on the injection of human PBMCs, culprit drugs, and MSCs as follows: 1) control (PBMCs
−drug
−MSCs
−); 2) PBMC-only (PBMCs
+drug
-MSCs
−); 3) drug-only (PBMCs
−drug
+MSCs
−); 4) SJS/TEN model mice treated with MSCs (PBMCs
+drug
+MSCs
+); and 5) lamotrigine-induced SJS/TEN model mice (PBMCs
+drug
+MSCs
−). Drug-specific human PBMCs (1.5 × 10
6 cells/mouse) were injected intravenously into the mice. Subsequently, we administered lamotrigine orally once a day for 14 days (0.3 ng/g/day on days 1 to 7, and 0.6 ng/g/day on days 8 to 14).
To verify their therapeutic effects, MSCs (2.0 × 10
6 cells/mouse) derived from umbilical cord blood (gift from Asan Stem Cell Center) were transferred into NOG mice in the therapeutic group 14 days after the injection of PBMCs. Body weights and physical conditions, especially of the eye, were checked daily over the 26-day experimental period. At 25 days after the injection of PBMCs (i.e., 11 days after the MSC injection), photos of the eyes were taken under anesthesia just prior to sacrifice by cardiac puncture and blood collection (
Fig. 1).
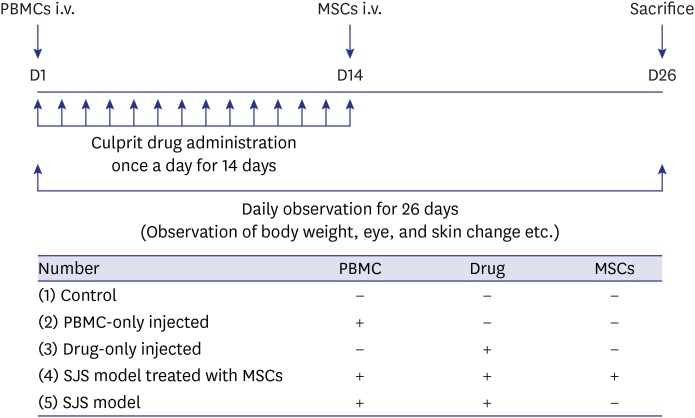 | Fig. 1
Summary of the experimental design and schedules. A lamotrigine-induced SJS mouse model was constructed using PBMCs from one patient and the culprit drug, lamotrigine. Effects of MSCs were compared by dividing groups into whether or not MSCs were injected.
SJS = Stevens-Johnson syndrome, PBMCs = peripheral blood mononuclear cells, MSCs = mesenchymal stem cells.

|
After sacrifice, mouse eyeballs were fixed in 4% paraformaldehyde for 24 hours, placed into 10% neutral buffered formalin for 4 hours, then incubated in 70% ethanol for 24 hours. The fixed eyeballs were embedded in paraffin, sectioned (3 µm), and mounted onto microscope slides for histochemical staining. Hematoxylin and eosin, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), and periodic acid-Schiff (PAS) staining were performed on the samples. TUNEL-negative cells were a dark blue and TUNEL-positive cells a dark brown color. PAS staining revealed goblet cells in the lining of the conjunctiva that were a dark purple color, which was considered PAS-positive. Tissue sections (5 µm) were mounted onto coated slides, and immunohistochemical staining for granzyme B (1:300 dilution), and 3,3-diaminobenzidine staining, were performed. Images were taken using an Olympus camera.
First, a histopathological analysis was conducted to determine the effect of MSCs on the SJS model. In the groups with no treatment, PBMC treatment, or drug only treatment (i.e., negative control groups 1–3), the eyeballs exhibited normal tissue structures. However, lamotrigine-induced SJS model mice treated with PBMCs and drugs but without MSCs (group 5) exhibited severe damage to the shapes of the corneas, limbus, nuclear layers, and eyelids. In contrast, for mice that received PBMCs, drugs, and MSCs (group 4), the shapes of the corneas, limbus, nuclear layers, and eyelids were comparable to those in the negative control groups (groups 1–3). These findings indicated beneficial therapeutic effects of the MSCs. In addition, the corneal opacity grade increased and the shape of the limbus was distorted in group 5. However, mice receiving MSCs (group 4) had decreased opacity grades and a normal limbus shape similar to those mice in groups 1–3 (
Fig. 2A and B).
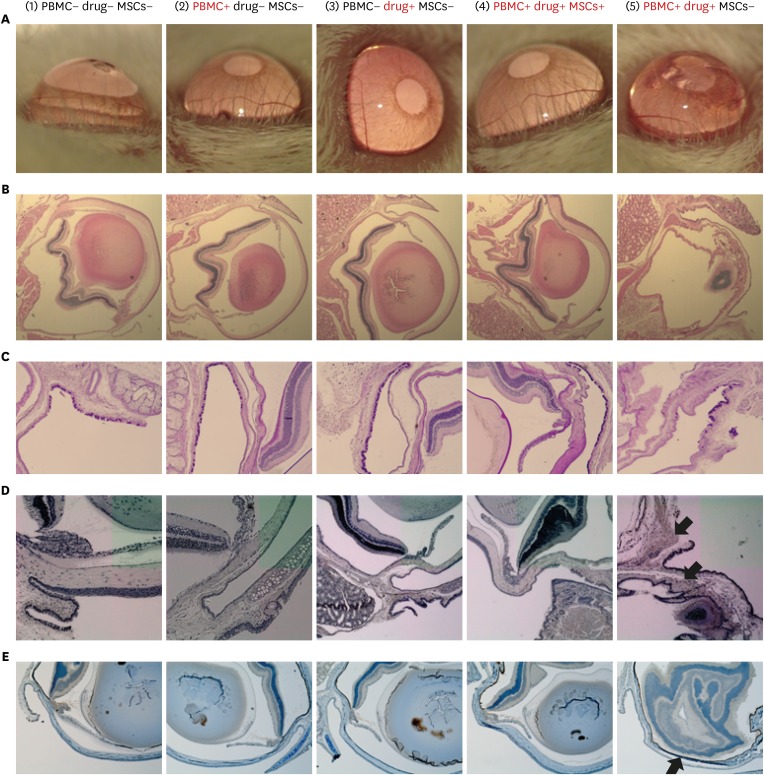 | Fig. 2
MSCs reduce mucosal damage in a lamotrigine-induced SJS/TEN mouse model. (A) Ocular findings were obtained using an operating microscope. The corneal opacity grade is increased in group 5. (B) Hematoxylin and eosin staining. The shape of the limbus is distorted in group 5; however, mice receiving MSCs (group 4) show a normal limbus. (C) PAS staining. The proportion of PAS-positive cells (dark purple color) observed in group 4 is shown. (D) Terminal deoxynucleotidyl transferase dUTP nick end labeling. (E) Immunohistochemical granzyme B staining. The entire inner side of the cornea is stained positively (dark brown) for granzyme B. Group 1: untreated control; Group 2: PBMCs-only; Group 3: lamotrigine-only; Group 4: Model mice (PBMCs + lamotrigine) treated with MSCs; Group 5: Model mice (PBMCs + lamotrigine) without MSC treatment.
PBMCs = peripheral blood mononuclear cells, MSCs = mesenchymal stem cells, SJS/TEN = Stevens-Johnson syndrome and toxic epidermal necrolysis, PAS = Periodic acid-Schiff.

|
Second, we performed PAS staining to compare mucosal damage in conjunctiva among the groups. The negative control groups (groups 1–3) had a high density of PAS-positive cells in their conjunctiva. Conversely, the proportion of PAS-positive cells was significantly decreased in lamotrigine-induced SJS model mice (group 5), whereas in mice treated with MSCs (group 4), the number of goblet cells was maintained (
Fig. 2C).
Subsequently, we analyzed cellular apoptosis using TUNEL staining. Apoptosis markedly increased in lamotrigine-induced SJS model mice (
Fig. 2D). To determine whether granzyme activation affected SJS/TEN, we stained cells for granzyme B. Notably, the negative control and MSC therapy groups had no granzyme B-positive cells in their conjunctiva. Conversely, in lamotrigine-induced SJS model mice, a high proportion of granzyme B-positive cells was identified in the inner lining of their conjunctiva (
Fig. 2E).
In the present study, we demonstrated a therapeutic effect of MSCs on SJS/TEN model mice. This model was generated using human PBMCs and a causative drug (lamotrigine), and MSCs were injected intravenously as a therapeutic candidate. The results suggest that MSCs mediate functional recovery of damaged ocular structures in SJS/TEN model mice. Based on these findings, we propose that MSCs prevent tissue destruction by modulating immune responses or regeneration/repair of tissues in SJS/TEN. Although further studies are required to determine the precise effects of MSCs on SJS/TEN, these cells could provide a novel therapy for the management of these disorders.
To date, there has been no research on the therapeutic effects of systemic injection of MSCs in SJS/TEN. Several studies have been undertaken on ocular disease in an inflammation-induced dry eye mouse model, in which the opacity grades decreased in ethanol-damaged corneas and the number of goblet cells recovered following treatment with MSCs.
1011 We also demonstrated that granzyme B was involved in the pathophysiology of the present mouse model. While previous studies suggested that granzyme B was a potential damaging factor in SJS/TEN, the mechanisms underlying site-selective apoptosis were yet to be elucidated.
1 In a recent study, synergistic activation of perforin and granzyme B was observed using live-cell imaging.
1 Upon activation, perforin and granzyme B move to specific sites and perforin forms pores in the target membrane, resulting in the influx of granzyme B and apoptosis in the region.
1 In the present study, SJS model mice without MSC treatment had a high proportion of granzyme B-positive cells, whereas granzyme B-positive cells were rare in the MSC-treated groups. These results indirectly demonstrate the anti-inflammatory functions of MSCs.
Because SJS/TEN is a very rare disease, it was difficult to obtain samples from patients presenting typical SJS/TEN clinical manifestations associated with a specific culprit drug over a defined period. Thus, a limitation of the present study was that the experiment was conducted using a blood sample from only one patient with SJS. In addition, we could not observe skin exfoliation and bullous changes in the SJS/TEN mouse model. The mouse model has an epithelium of murine origin and drug-specific lymphocytes have a human origin; therefore, skin manifestations do not occur unless human epithelium is transplanted. However, when transplanting human tissue into a mouse it is difficult to distinguish graft-versus-host disease reactions from SJS progression. The ocular lesion occurred first in the SJS-TEN mouse model at 2 weeks, and it was impossible to observe more than two clinical features in one mouse. Because of these limitations, our findings will need to be confirmed by additional experiments with more samples and other drugs.
Nevertheless, the present study is crucial for identifying a potential SJS/TEN therapy as it is the first to demonstrate the effectiveness of intravenous injection of MSCs in a mouse model of SJS/TEN. Although the results do not reveal the mechanism of stem cell action, it is a major landmark study that could facilitate further studies regarding MSCs that focus on treatments for SJS/TEN.
ETHICS STATEMENT
The study was performed in accordance with the Declaration of Helsinki, and the protocols were approved by the Institutional Review Board of Asan Medical Center (approval No. 2016-1083) and the Institutional Animal Care and Use Committee of Asan Institute for Life Science (2016-1083). The patient provided written informed consent.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download