INTRODUCTION
METHODS
Study populations
Measurement of serum 25(OH)D3
Diagnostic criteria and definition
Statistics
Ethics statement
RESULTS
Baseline characteristics of patients
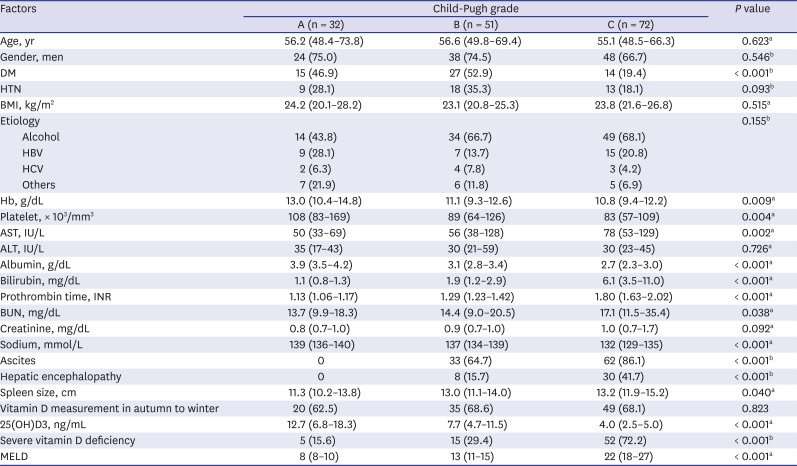
Table 1
Baseline characteristics based on Child-Pugh grades
Serum 25(OH)D3 levels as a representative for liver function
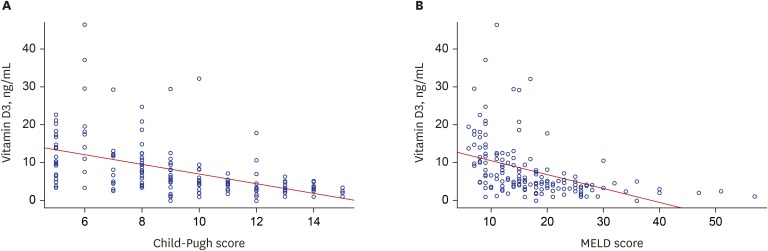 | Fig. 1Correlation between serum 25(OH)D3 levels and prognostic models for liver cirrhosis. (A) Correlation between serum levels and Child-Pugh scores and (B) MELD scores. Serum 25(OH)D3 levels were inversely correlated with Child-Pugh scores (Pearson correlation coefficient, −0.493; P < 0.001) and MELD scores (Pearson correlation coefficient, −0.436; P < 0.001).25(OH)D3 = 25-hydroxyvitamin D3, MELD = Model for End-stage Liver Disease.
|
Vitamin D deficiency as an independent predictor for survival
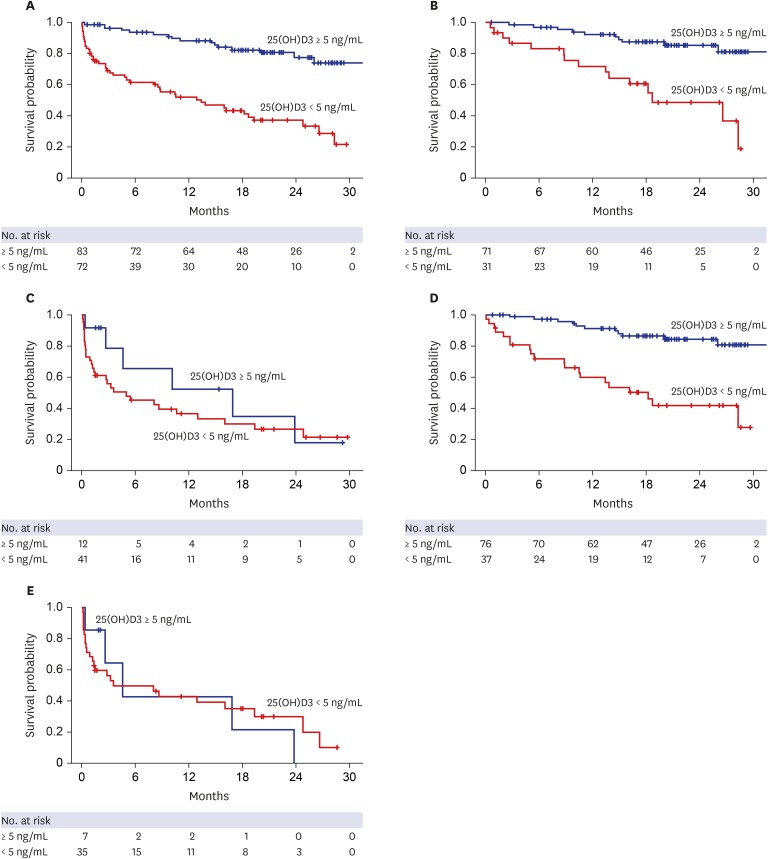 | Fig. 2Kaplan–Meier plots for primary outcomes based on the presence of severe vitamin D deficiency and MELD scores. (A) LT-free survival rates based on the presence of severe vitamin D deficiency (median survival time, not reached vs. 12.9 months for patients without and with severe vitamin D deficiency, respectively; P < 0.001). (B) LT-free survival rates in patients with Child-Pugh scores of 5–10 (median survival time, not reached vs. 18.7 months; P < 0.001). (C) LT-free survival rates in patients with Child-Pugh scores of 11–15 (median survival time, 16.8 vs. 4.9 months; P = 0.320). (D) LT-free survival rates in patients with a MELD score of ≤ 20 (median survival time, not reached vs. 16.2 months; P < 0.001). (E) LT-free survival rates in patients with a MELD score of > 20 (median survival time, 9.5 vs. 8.1 months, respectively; P = 0.890).LT = liver transplantation, MELD = Model for End-stage Liver Disease, 25(OH)D3 = 25-hydroxyvitamin D3.
|
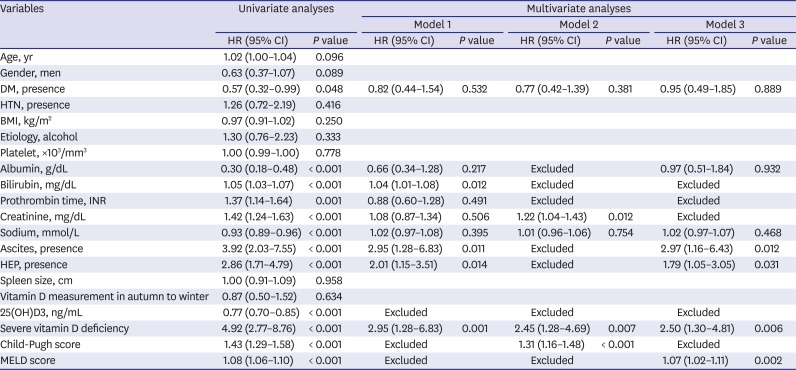
Table 2
Univariate and multivariate Cox regression analyses for mortality and urgent transplantation
Vitamin D deficiency and survival in relation to Child-Pugh or MELD scores
Predictive power of a combination model containing MELD score and vitamin D deficiency
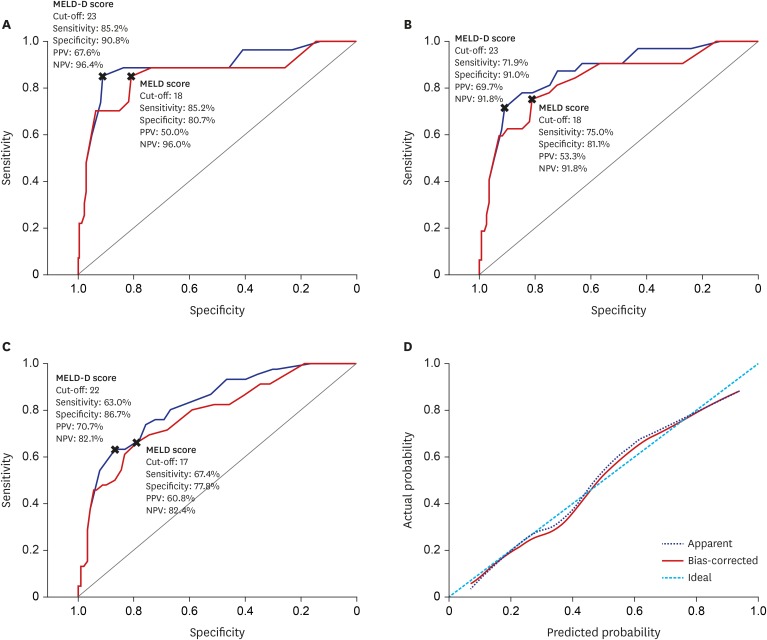 | Fig. 3Comparing predictivity of MELD-D and MELD scores for short- and long-term survival. (A) AUROC curves for 3-month mortality. (B) AUROC curves for 6-month mortality. (C) AUROC curves for 12-month mortality. MELD-D scores had significantly higher AUCs than MELD scores through 3-,6-,12-months (AUC: 0.889 vs. 0.855, 0.870 vs. 0.833, and 0.823 vs. 0.782; P = 0.018, 0.011, and 0.009, respectively). (D) Calibration plot for 12-month mortality. The mean absolute error was 0.019 using 40 repetitions of bootstrap.MELD-D = Model for End-stage Liver Disease with vitamin D, MELD = Model for End-stage Liver Disease, AUROC = area under the receiver operating characteristic curve, AUC = area under the curve, PPV = positive predictive value, NPV = negative predictive value.
|
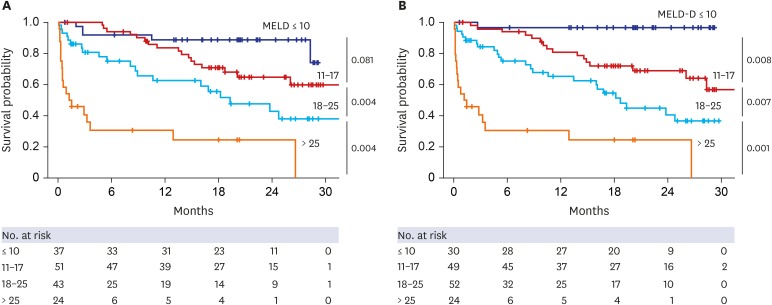 | Fig. 4Kaplan–Meier plot for primary outcome according to four categories of MELD and MELD-D scores. (A) MELD and (B) MELD-D scores were stratified into four categories: ≤ 10, 11–17, 18–25, and > 25. These categories resulted from the optimization process of MELD categorization. Cumulative survival rates during the follow-up period were globally more differentiated according to categories in MELD-D scores than MELD scores. Especially, between categories of ≤ 10 and 11–17, MELD-D scores significantly discriminated survival, but MELD scores did not (P = 0.008 vs. 0.081). The numbers mentioned to the right of each graph indicate P values between groups.MELD = Model for End-stage Liver Disease, MELD-D = Model for End-stage Liver Disease with vitamin D.
|
Serum 25(OH)D3 level as a risk factor for cirrhosis complications
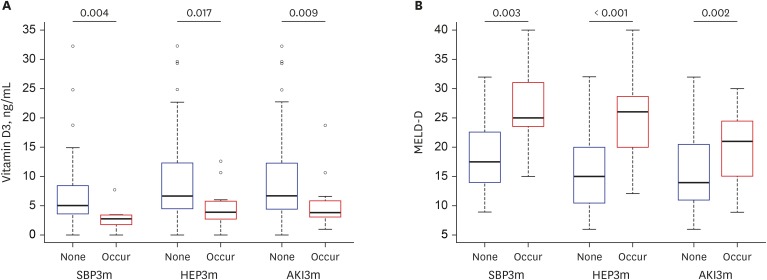 | Fig. 5Levels of serum 25(OH)D3 and MELD-D based on the new occurrence of cirrhotic complications at baseline and within three months. (A) Levels of serum 25(OH)D3 according to new occurrences of cirrhotic complications, including SBP, HEP, and AKI. (B) MELD-D scores according to new occurrences of cirrhotic complications. The numbers above the thick lines at the top of graphs indicate P values between the patients with and without each complication, respectively. Lower 25(OH)D3 and higher MELD-D scores were associated with new occurrences of cirrhotic complications within three months.25(OH)D3 = 25-hydroxyvitamin D3, MELD-D = Model for End-stage Liver Disease with vitamin D, SBP = spontaneous bacterial peritonitis, HEP = overt hepatic encephalopathy, AKI = acute kidney injury.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download