1. Park SM, Shen F, Kim HJ, Kim H, Chang BS, Lee CK, et al. How many screws are necessary to be considered an experienced surgeon for freehand placement of thoracolumbar pedicle screws?: analysis using the cumulative summation test for learning curve. World Neurosurg. 2018; 118:e550–6. PMID:
30257308.

2. Perna F, Borghi R, Pilla F, Stefanini N, Mazzotti A, Chehrassan M. Pedicle screw insertion techniques: an update and review of the literature. Musculoskelet Surg. 2016; 100(3):165–169. PMID:
27866324.

3. Esses SI, Sachs BL, Dreyzin V. Complications associated with the technique of pedicle screw fixation. A selected survey of ABS members. Spine. 1993; 18(15):2231–2238. PMID:
8278838.

5. Song G, Bai H, Zhao Y, Han J, Liu X. Preoperative planning and simulation for pedicle screw insertion using computed tomography-based patient specific volume rendering combined with projection fluoroscopy. Int Robot Autom J. 2017; 2(1):25–29.

6. Xiang L, Zhou Y, Wang H, Zhang H, Song G, Zhao Y, et al. Significance of preoperative planning simulator for junior surgeons' training of pedicle screw insertion. J Spinal Disord Tech. 2015; 28(1):E25–E29. PMID:
25075987.

7. Yeom JS, Choy WS, Kim WJ, Kim HY, Kang JW, Kim YW, et al. A patient-specific surgical simulation system for spinal screw insertion composed of virtual roentgenogram, virtual C-arm, and rapid prototyping. J Korean Orthop Assoc. 2001; 36(2):161–166.

8. Park SK, Yeom JS, Won JH, Lee JC, Lee DH, Lee J, et al. C1-2 fixation using polyaxial screws and rods assisted by computer simulation for revision of failed posterior fusion: a technical report. J Korean Orthop Assoc. 2005; 40(6):778–781.

9. Eftekhar B, Ghodsi M, Ketabchi E, Rasaee S. Surgical simulation software for insertion of pedicle screws. Neurosurgery. 2002; 50(1):222–223. PMID:
11844256.

10. Muralidharan V, Swaminathan G, Devadhas D, Joseph BV. Patient-specific interactive software module for virtual preoperative planning and visualization of pedicle screw entry point and trajectories in spine surgery. Neurol India. 2018; 66(6):1766–1770. PMID:
30504578.

11. Klein S, Whyne CM, Rush R, Ginsberg HJ. CT-based patient-specific simulation software for pedicle screw insertion. J Spinal Disord Tech. 2009; 22(7):502–506. PMID:
20075813.

12. Rajon DA, Bolch WE. Marching cube algorithm: review and trilinear interpolation adaptation for image-based dosimetric models. Comput Med Imaging Graph. 2003; 27(5):411–435. PMID:
12821034.

13. Raj Ségonne F, Fischl B. Integration of topological constraints in medical image segmentation. In : Paragios N, Duncan J, Ayache N, editors. Handbook of Biomedical Imaging. Boston, MA: Springer;2015. p. 245–262.
14. Wang J, Yu Z. Feature-sensitive tetrahedral mesh generation with guaranteed quality. Comput Aided Des. 2012; 44(5):400–412. PMID:
22328787.

15. Zhang Y, Hughes TJ, Bajaj CL. An automatic 3D mesh generation method for domains with multiple materials. Comput Methods Appl Mech Eng. 2010; 199(5-8):405–415. PMID:
20161555.

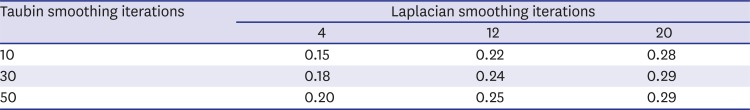
16. Vollmer J, Mencl R, Müller H. Improved laplacian smoothing of noisy surface meshes. Comput Graph Forum. 1999; 18(3):131–138.

17. Manmadhachary A, Ravi Kumar Y, Krishnanand L. Improve the accuracy, surface smoothing and material adaption in STL file for RP medical models. J Manuf Process. 2016; 21:46–45.
18. Chartrand G, Cresson T, Chav R, Gotra A, Tang A, De Guise JA. Liver segmentation on CT and MR using Laplacian mesh optimization. IEEE Trans Biomed Eng. 2017; 64(9):2110–2121. PMID:
27893375.

19. Wang LC, Hung YC. Hole filling of triangular mesh segments using systematic grey prediction. Comput Aided Des. 2012; 44(12):1182–1189.

20. Sazonov I, Nithiarasu P. Semi-automatic surface and volume mesh generation for subject-specific biomedical geometries. Int J Numer Methods Biomed Eng. 2012; 28(1):133–157.

21. Jermyn M, Ghadyani H, Mastanduno MA, Turner W, Davis SC, Dehghani H, et al. Fast segmentation and high-quality three-dimensional volume mesh creation from medical images for diffuse optical tomography. J Biomed Opt. 2013; 18(8):86007. PMID:
23942632.

22. Kalaiselvi T, Sriramakrishnan P, Somasundaram K. Survey of using GPU CUDA programming model in medical image analysis. Inform Med Unlocked. 2017; 9:133–144.

23. Zhou K, Gong M, Huang X, Guo B. Data-parallel octrees for surface reconstruction. IEEE Trans Vis Comput Graph. 2011; 17(5):669–681. PMID:
20498507.
24. Garland M, Zhou Y. Quadric-based simplification in any dimension. ACM Trans Graph. 2005; 24(2):209–239.

25. Papageorgiou A, Platis N. Triangular mesh simplification on the GPU. Vis Comput. 2015; 31(2):235–244.

26. Matias van Kaick O, Pedrini H. A comparative evaluation of metrics for fast mesh simplification. Comput Graph Forum. 2006; 25(2):197–210.

27. Rypl D, Nerad J. Volume preserving smoothing of triangular isotropic three-dimensional surface meshes. Adv Eng Softw. 2016; 101:3–26.

28. Bian Z, Tong R. Feature-preserving mesh denoising based on vertices classification. Comput Aided Geom Des. 2011; 28(1):50–64.

29. Petitjean S. A survey of methods for recovering quadrics in triangle meshes. ACM Comput Surv. 2002; 34(2):211–262.

30. Gosselin MC, Neufeld E, Moser H, Huber E, Farcito S, Gerber L, et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: the Virtual Population 3.0. Phys Med Biol. 2014; 59(18):5287–5303. PMID:
25144615.

31. Deese K, Geilen M, Rieg R. A two-step smoothing algorithm for an automated product development process. Int J Simul Model. 2018; 17(2):308–317.

32. Chen CY, Cheng KY. A sharpness dependent filter for mesh smoothing. Comput Aided Geom Des. 2005; 22(5):76–391.

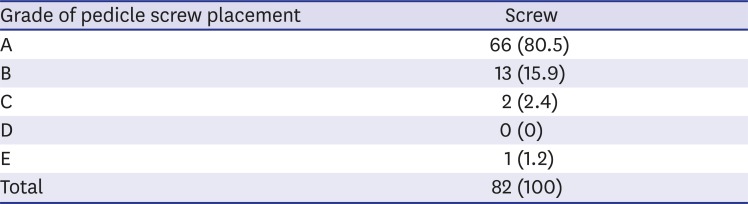
33. Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine. 1990; 15(1):11–14. PMID:
2326693.

34. Tang M, Lee M, Kim YJ. Interactive Hausdorff distance computation for general polygonal models. ACM Trans Graph. 2009; 28(3):74.

35. Bonaretti S, Seiler C, Boichon C, Reyes M, Büchler P. Image-based vs. mesh-based statistical appearance models of the human femur: implications for finite element simulations. Med Eng Phys. 2014; 36(12):1626–1635. PMID:
25271191.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download