INTRODUCTION
Fragility fracture has been a significant public health issue since the prevalence of osteoporosis has increased with the aging population. The number of elderly worldwide is expected to more than double to 2 billion, with 8.9 million fragility fractures occurring annually by 2050.
1 In this aging society, the socioeconomic burden due to fragility fracture is expected to increase significantly. Among fragility fractures, hip fractures have demonstrated high mortality rates and their resultant disability has been well documented. However, vertebral fractures are often silent and not recognized.
2 Therefore, it is difficult to assess the exact incidence and its direct socioeconomic effects.
A vertebral fragility fracture (VFF) is a major cause of increased back pain, decreased quality of life, and increased mortality in the elderly.
3 Choi et al.
4 reported that osteoporotic vertebral fractures occurred 852.24 cases per 100,000 people in Korea, and that number is increasing year by year. To prevent disability related with VFF, early diagnosis and prevention of osteoporosis have been emphasized.
567 Considering that the incidence of VFF rapidly increases with age among women over 50 years old, it is reasonable to say that the most important goal of osteoporosis treatment is to prevent the first vertebral fracture.
7 However, anti-osteoporosis medications are commonly recommended for elderly patients (≥ 65 years old) with osteoporosis or after a fragility fracture in most public health systems worldwide.
8
Dual energy X-ray absorptiometry (DXA) scans are standard methods to determine bone mineral density (BMD), and BMD is the only standard that allows most public health authorities to decide whether to start anti-osteoporosis medication before fractures occur.
9 However, there have been under-treatment problems, so called the osteoporosis treatment gap,
10 recognized as a serious public health issue.
11 Further, physicians often encounter VFFs in patients classified as having osteopenia or even in patients with normal bone density.
12
The study began with questions about the discrepancy between VFF and BMD. We investigated the characteristics of patients with VFF and the correlation between the age-related incidence of VFF and BMD. Furthermore, we analyzed other factors affecting the occurrence of VFF and the annual anti-osteoporosis prescription rates.
Go to :

METHODS
This study was a retrospective cross-sectional study. Patients who were diagnosed with vertebral fracture and treated conservatively in a single tertiary hospital from January 2005 to December 2016 were selected. Conservative treatment included a first period of bed rest followed by gradual mobilization with brace. The inclusion criteria were as follows: 1) Patients over 50 years old who had been diagnosed with International Statistical Classification of Diseases and Related Health Problems, 10th edition (ICD-10) codes of S22, S32, or M80, including thoracolumbar fractures and osteoporotic compression fractures, and had radiographic imaging to identify the fractures; and 2) patients who had BMD data by DXA scan at the time of diagnosis. Exclusion criteria included: 1) Patients who did not have BMD data; 2) incomplete imaging to identify the fracture or missing clinical assessment in the medical record; and 3) patients with secondary osteoporosis, which was defined as osteoporosis resulting from medical disorders, including endocrine disorders, adverse effects of steroid medications, chronic renal disease, and cancer (
Fig. 1).
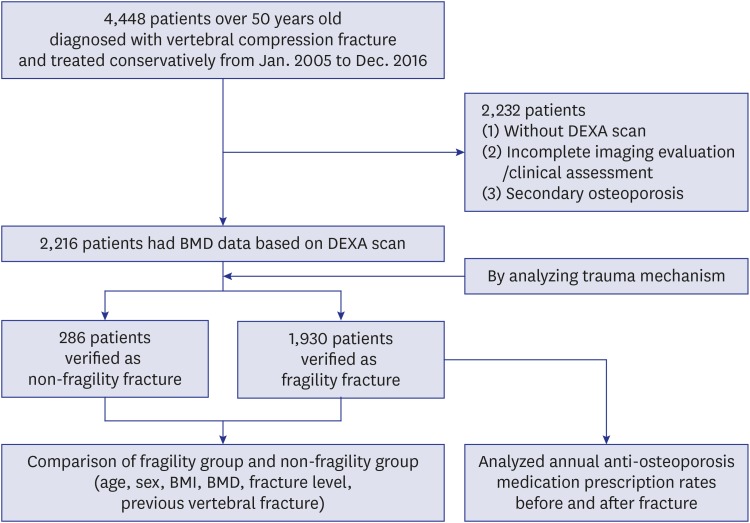 | Fig. 1
Flow chart of the study.
DXA = dual energy X-ray absorptiometry, BMD = bone mineral density, BMI = body mass index.

|
For subgroup analysis, patients were divided into fragility and non-fragility groups according to their injury mechanism in medical records. A fragility fracture was defined as a fracture caused by an injury like a fall from a standing height or less, which would not normally result in a fracture, or from no identifiable trauma.
13 Patients were divided by age (50–59, 60–69, 70–79, and
> 80 years old) and we defined those under 70 as the ‘younger group’ and those 70 and over as the ‘older group.’ Patients' age, gender, body mass index (BMI), BMD, fracture level, previous vertebral fracture, and history of anti-osteoporosis medication were obtained by reviewing medical records. Anti-osteoporosis medication before and after fracture included bisphosphonate, selective estrogen receptor modulator, and/or parathyroid hormone, and the survey of prescription rate included even a one-time prescription event after fracture.
As a part of the radiographic diagnosis of the vertebral fracture, two orthopedic surgeons (with 3 years and 5 years of experience) independently reviewed imaging studies including simple radiographs, computed tomography, or magnetic resonance imaging, using the picture archiving and communicating system and identified the number and levels of the fractured vertebrae with reference to medical records. Disagreements between the two evaluators were resolved by discussion.
BMD was measured at total hip, femoral neck, and lumber spine (L1–L4) regions by trained technicians using DXA (Lunar Prodigy densitometer; GE Healthcare, Madison, WI, USA). Osteopenia or osteoporosis was diagnosed with reference BMD values using the T-score criteria of the World Health Organization (WHO): Normal, T-score of total hip, femoral neck and lumbar spine at any site ≥ −1; Osteopenia, −2.5 < the lowest T-score < −1; Osteoporosis, the lowest T-score ≤ −2.5.
11 The lowest T-score among three regions was selected as the overall value.
14 All evaluable vertebrae were used but those distressed by local structural changes (e.g., metallic object, large osteophytes, fracture, surgery, scoliosis, etc.) were excluded from the analysis. If a T-score of a vertebra differed by more than 1.0 from the adjacent vertebra, it was excluded from the mean value determination.
15 Also, if only one evaluable vertebra remained after excluding other deformed vertebrae, the patient was excluded from the study because the T-score could not be calculated according to the International Society for Clinical Densitometry (ISCD) guidelines.
16
Statistical analysis
The differences between the two groups were examined using χ2 for categorical variables (gender, WHO osteoporosis classification, previous vertebral fracture) and independent t-tests for continuous variables (age, BMI, BMD, number of fractured vertebrae). The trend of the proportion of fragility fracture by age groups was analyzed using linear by linear association. The odds ratio for VFF occurrence in relation to BMD values was also calculated using Nagelkerke R-squared regression analysis. The WHO osteoporosis classification and BMD results were used as the independent variables and age and gender were amended in each analysis. To identify the predictive factors for VFF by age groups, multiple logistic regression analysis was used. Factors that had univariate P values of < 0.1 were included for multivariate analysis. All statistical analyses were performed using SPSS version 23.0 (SPSS, Chicago, IL, USA).
Ethics statement
The study was approved by the ethical review board of Chung-Ang University Hospital, under protocol no. 1702-007-16041. For this type of study formal consent is not required.
Go to :

DISCUSSION
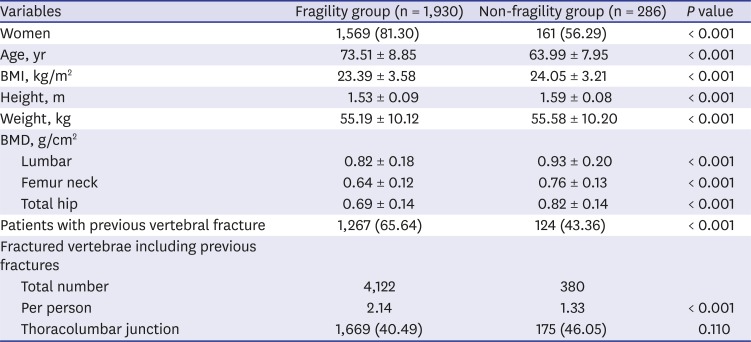
The fragility group demonstrated a statistically higher women ratio, older age, lower BMI, lower BMD values in all three regions, and a greater incidence of previous vertebral fractures than those of the non-fragility group. Women accounted for 81.30% of the fragility group, which is similar to the ratio seen in the study conducted by Vanasse et al.
17 Their cohort, 77% of which was women, consisted of 25,852 individuals with fragility fractures. In our study, as the predictive factor of VFF, women had a greater odds ratio in the younger group than in the older group. A lower BMI is usually considered a risk factor for osteoporosis and fragility fracture; however, the relationship between BMI and fracture could be controversial. In this study, BMI was not a statistically significant predictive factor for VFF. Different studies have shown a protective role of obesity against osteoporosis, but recent evidence suggests that obesity, and thus fat mass, may prove to be risk factors for decreased bone density and fractures.
18
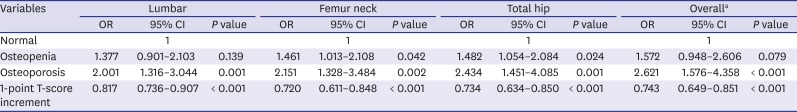
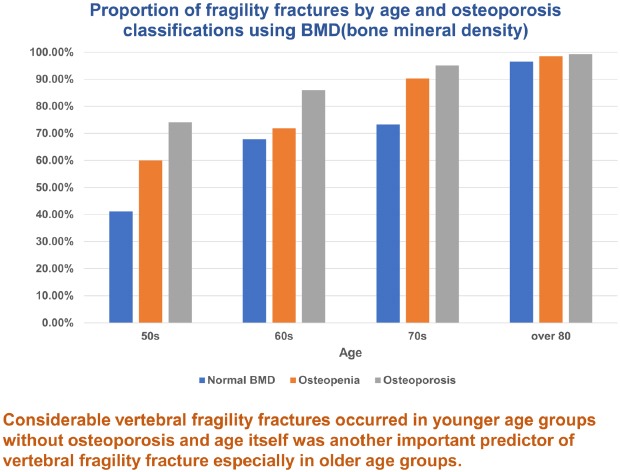
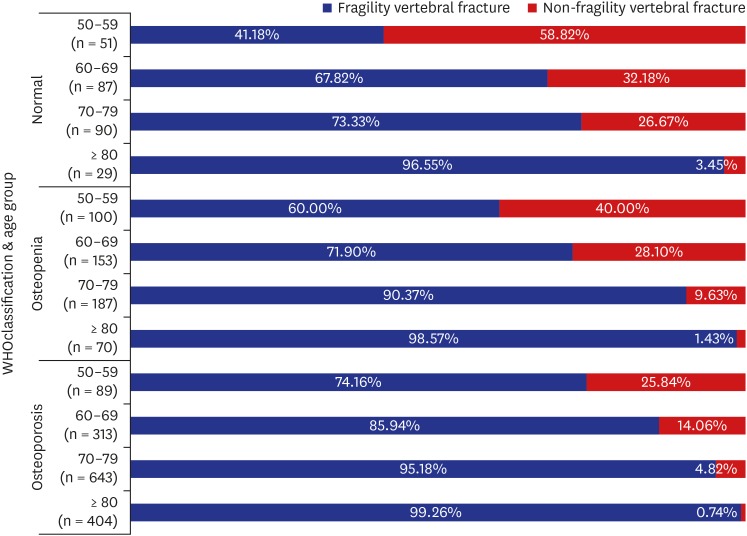
As seen in this study, lower BMD is a well-known potent risk factor for VFF. In our results, the patients classified as having osteopenia and osteoporosis had 1.57 and 2.62 fold higher risk of VFFs than patients with normal bone density, respectively. And an increase of 1 point in overall T-score prevented 36% of VFF in the older group and 27% of VFF in the younger group. This suggests that BMD and the WHO osteoporosis classification is useful for predicting the relative risk. However, even in the patients classified as normal and osteopenia, 41.18% and 60.00% in their 50s, 67.82% and 71.90% in their 60s, 73.33% and 90.37% in their 70s, and 96.55% and 98.57% in their 80s or older had fragility fractures, respectively. Such findings mean that the BMD value alone does not accurately reflect the risk of VFF. Siris et al.
12 also reported that more than 80% of postmenopausal women with fragility fractures had osteopenia or normal findings, and other studies support the poor accuracy of BMD in predicting osteoporotic fractures.
19 Therefore, even if the BMD value is in normal range, prevention and treatment of osteoporosis should not be neglected.
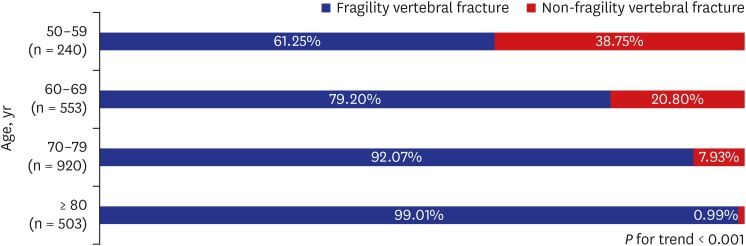
Above all, our results showed that the proportion of VFF increased consistently with age and the tendency was constant in all groups divided by WHO osteoporosis classification. A recent epidemiologic study reported that vertebral fractures start to increase from sixty years of age.
20 A meta-analysis reported that BMD values at the lumbar spine and femoral neck decreased by 0.023 g/cm
2 and 0.041 g/cm
2 for men and 0.063 g/cm
2 and 0.054 g/cm
2 for women per decade.
21 In our study, the proportion of fragility fractures was more than 73% in patients older than 70 years and more than 97% in those older than 80 years, even in patients with normal BMD. These findings support that age itself as well as BMD could be another important risk factor for VFF.
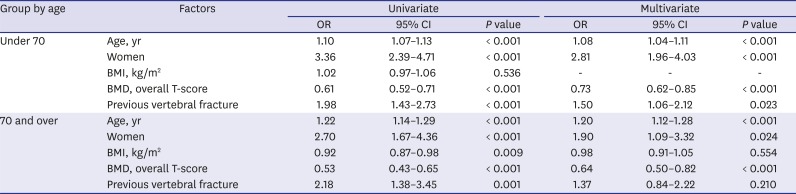
As a result of multivariate analysis to evaluate the predictive factor of VFF occurrence, the odds ratio of age in the older group was 1.20, which was greater than 1.09 in the younger group. In the younger group, an increase in age of five increases the risk of VFF by about 1.5-fold, and in the older group by about 2.5-fold. This means that the effect of age in the older group was greater than in the younger group.
As noted, age is a key determinant for public health authorities to treat osteoporosis and the ISCD guidelines recommend screening at age 65 or older. However, our study reveals that a large proportion of people in their 50s experience VFF in all groups divided by WHO osteoporosis classification. These findings strongly support the need to change the criteria for screening or supplement tools for determining clinical risk factors.
Our results demonstrated a higher incidence of previous vertebral fracture (65.64%) in the fragility group; however, previous vertebral fractures were also seen in 43.36% of the non-fragility group. In contrast to fractures occurring at other skeletal sites, vertebral fractures occasionally occur silently, and two-thirds to three-quarters of these are not recognized at the time of their clinical occurrence.
2 Delmas et al.
22 demonstrated worldwide under-diagnosis of vertebral fractures in women over 65 years old with osteoporosis, with false negative rates of 45.2% in North America, 46.5% in Latin America and 29.5% in Europe/South Africa/Australia. These prevalent vertebral fractures might be the most important factor in predicting subsequent vertebral fracture.
23 Similarly, our results show that the presence of previous vertebral fractures, especially in the younger group, was a predictor of VFF. In contrast, wrist and forearm fractures are common osteoporotic fractures that occur at a relatively young age, but these fractures do not appear to well represent fragility fracture considering that the incidence was not found to increase in the elderly and the fractures are closely correlated with activity regardless of age.
24 As the purpose of proactive osteoporosis treatment is to prevent the first vertebral fracture, VFF itself should be considered as an indication for osteoporosis treatment.
25 Further, spine lateral radiography, especially of the thoracolumbar area, could be very helpful as a screening tool to start active anti-osteoporosis medication regardless of BMD value.
Marshall et al.
26 compared the relative increase in fracture in women for each decrease in BMD measured at different sites including the distal radius, femoral neck, and lumbar spine. They reported that a decrease in femoral neck BMD best reflected the relative risk for all fractures. Another study also stated that the total hip appears to be the optimal site for BMD measurement to predict osteoporotic fracture.
27 The lumbar spine is not the best diagnostic site because lumbar BMD seems to be easily affected by various conditions such as degenerative changes, fractures, and obesity, etc. In our study, the change of T-scores in hip regions (femur neck and total hip) had a relatively greater impact on the occurrence of fragility fracture than score changes in the lumbar region (
Table 2). Decreases in hip BMD should be considered as a more serious sign for VFF risk than decreases in lumbar BMD.
Several observational studies have reported that under-treatment after vertebral fracture is still common, including a report by Kroth et al.
28 that less than 50% of patients received osteoporosis medications after vertebral fracture. Yusuf et al.
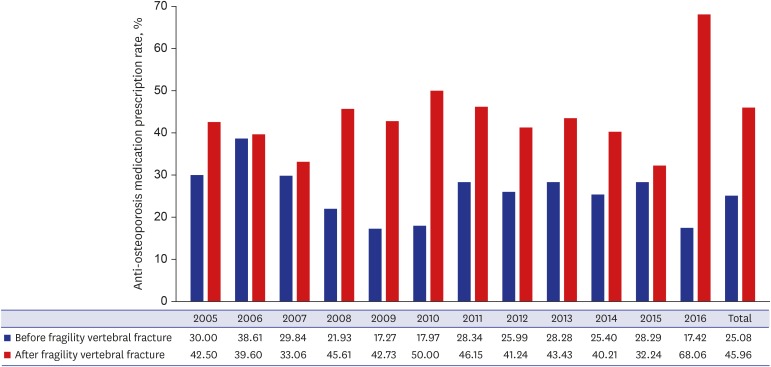
10 reported that less than one third of their cohort received an osteoporosis medication in the post-fracture year, when risk of a second fragility fracture is highest. In our study of 1,930 patients with fragility fractures, the average anti-osteoporosis medication prescription rates before and after fracture were 25.08% and 45.96%, respectively. Public health authorities can play a critical role in preventing such under-treatment, though individual physician awareness is also important. In our study, the anti-osteoporosis medication prescription rate after fracture increased abruptly in 2016 (
Fig. 4). In May 2015, a new National Health Insurance guideline came into effect specifying that patients with osteoporotic fractures with or without osteoporosis (T-score ≤ −2.5) would be treated by anti-osteoporosis medication for 3 years.
29 As a result, the prescription rate has increased. These changes highlight the importance of national health policies.
This study has several limitations. First, some selection bias might be inevitable due to the retrospective, cross-sectional design. Therefore, the collection of data or interpretation of the results also has limitations. For example, BMD values can be affected by osteoporosis medications, so patients who were treated for osteoporosis at the time of BMD measurement may have discrepancy between VFF and BMD. In addition, since the study population was limited to the patients diagnosed with vertebral fracture, the results and interpretation in this study may be different in the general population. Second, the fragility group had 1,930 patients while the non-fragility group had only 286 patients, so the difference in sample size between the two groups may have biased some statistical evaluations. Third, there was a possibility of inadequate assessment of the facts such as injury mechanism, history of anti-osteoporosis treatment, previous trauma, and other secondary osteoporosis causes, since the review was limited to records. For example, if a patient had been prescribed anti-osteoporosis medication in another hospital, this would not have been captured and it is likely that the patient was classified as not receiving an anti-osteoporosis prescription.
Our results demonstrated that a large proportion of patients with fragility fracture were not diagnosed with osteoporosis. Therefore, even if BMD is in the normal range, proactive treatment is needed if there are other risk factors identified in this study. Although osteoporosis has been identified as the main risk factors for vertebral fractures, age itself might be another important risk factor in patients over 70 years old. The discrepancy between the incidence of age-related VFF and BMD demonstrated in our study could suggest the necessity of supplemental screening tools and reconsideration of worldwide criteria (≥ 65 years old and osteoporosis, T-score ≤ −2.5) for starting anti-osteoporosis treatment.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download