INTRODUCTION
Encountering patients who need non-obstetric surgery during their pregnancy is always challenging to an anesthesiologist. The frequency of pregnancies with a need for a surgical procedure under anesthesia is known to be about 0.7%–2.0%.
12 In Korea, the rate has been reported at about 0.9%–2.5%.
34 Because it is difficult to conduct trials on pregnant women owing to safety and related ethical aspects, most studies adopted retrospective analyses methods. The recent research of 6.5 million pregnancies concluded that the risk associated with non-obstetric surgery was relatively low and surgical procedures during pregnancy are generally safe.
1 However, a majority of studies have reported higher incidences of adverse fetal outcomes in association with maternal surgeries and anesthetics during pregnancy.
5678910 The most recent study from Belgium reviewed records from a period of 16 years and found that surgeries during pregnancy were associated with more frequent preterm babies and lower birth weights.
11 Especially, as the most common non-obstetric surgery, appendectomies, were strongly associated with subsequent adverse fetal outcome.
710 Guidelines from the American College of Obstetrics and Gynecologists recommended that elective surgery should be delayed until after delivery and if possible, the surgery should be performed in the second trimester and emphasized an obstetric consultation before surgery and a team approach of anesthesiologists, obstetricians, and surgeons for optimal safety of the patient and the fetus.
12 Although modern techniques of surgery and development of anesthesia drugs contribute to diminishing fatal maternal outcome like maternal death,
17 it seems apparent that we cannot conclude that operations during pregnancy are safe, at least for now.
Here, we investigated the frequency and assessed the risk for adverse outcomes in women who underwent non-obstetric surgery during pregnancy at a single tertiary university hospital in the Asian population. Previous articles were mostly based on patients' registries and compared between cases of non-obstetric surgery and those who had no surgery. In this study, we focused on which surgical or maternal factors were related to a higher risk for adverse birth outcomes amongst the pregnant women who received a non-obstetric surgical procedure.
Go to :

METHODS
The study period ranged from January 2001 to November 2016. Two authors reviewed medical records of pregnant women who underwent non-obstetric surgery under general anesthesia or regional anesthesia in January 2017. Surgeries for an incompetent cervix or ectopic pregnancy, which were pregnancy-related surgeries, were excluded. Eventually, a total of 155 pregnant women were selected for the study (
Fig. 1).
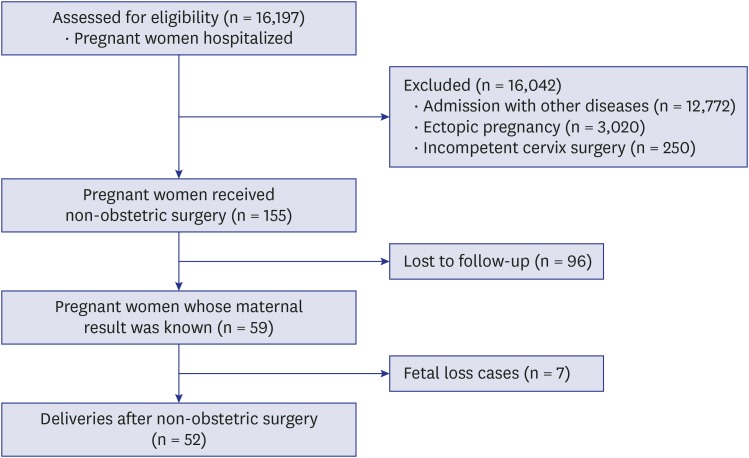 | Fig. 1 Flow chart of patients' enrollment.
|
Demographic data of patients, including age, height, weight, body mass index (BMI), gestational age, trimester, and gravida were collected from medical records. Anesthesia and surgery related data were also collected, including surgical site, type of anesthesia, and anesthesia and operation time. The surgical site was classified as abdominal and non-abdominal, and in case of abdominal surgery, it was classified as laparoscopic and laparotomy. Type of anesthesia was recorded as general or regional anesthesia, and in case of regional anesthesia, its subtype was also recorded. For patients who completed their antenatal care at our hospital, we collected data about the outcome of the pregnancy; the outcome assessment included the presence of preterm labor, premature birth, abortion, or stillbirth and the data of newborns including height, weight, and 1 minute and 5 minutes Apgar scores. We defined overall adverse outcome as the incidence of patients who experienced at least one of following: spontaneous abortion, threatened abortion (bloody vaginal discharge or bleeding through a closed cervical os during the first half of pregnancy), stillbirth, preterm labor, and premature birth.
The core protocol of general anesthesia for a pregnant woman in our hospital had not been changed for the study period. General anesthesia was inducted with glycopyrrolate, thiopental sodium, fentanyl, and rocuronium and maintained with sevoflurane, the mixture of air and oxygen, and intermittent fentanyl injection. Bispectral index monitoring was usually applied after it was commercialized. As reversal agents from neuromuscular blocking, we used glycopyrrolate and pyridostigmine. In the case of regional anesthesia, bupivacaine was used in spinal anesthesia, and levobupivacaine or ropivacaine was used in epidural anesthesia for combined spinal-epidural anesthesia. When we needed to do intravenous regional block, we used 0.5% lidocaine.
We compared variables to assess overall adverse outcome. Maternal demographic data, gestational age at surgery and delivery, duration of operation and anesthesia, and data of baby (Apgar scores, weight, and height of babies) were analyzed. In the case of surgery involving the abdomen, the analyses for comparison between laparoscopy and laparotomy were performed amongst these patients. Continuous variables were compared using Student's t-test, and categorical variables were analyzed using χ2 analysis or Fisher's exact test. Multiple logistic regression test was performed to identify predictors of overall adverse outcome. All data management and statistical analyses were done using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). All tests were considered statistically meaningful if the P value was less than 0.05.
Ethics statement
This retrospective study was approved by the Institutional Review Board (IRB) of Ewha Womans University Medical Center Mokdong Hospital (EUMC2016-12-011), and the IRB waived the need for informed consent.
Go to :

RESULTS
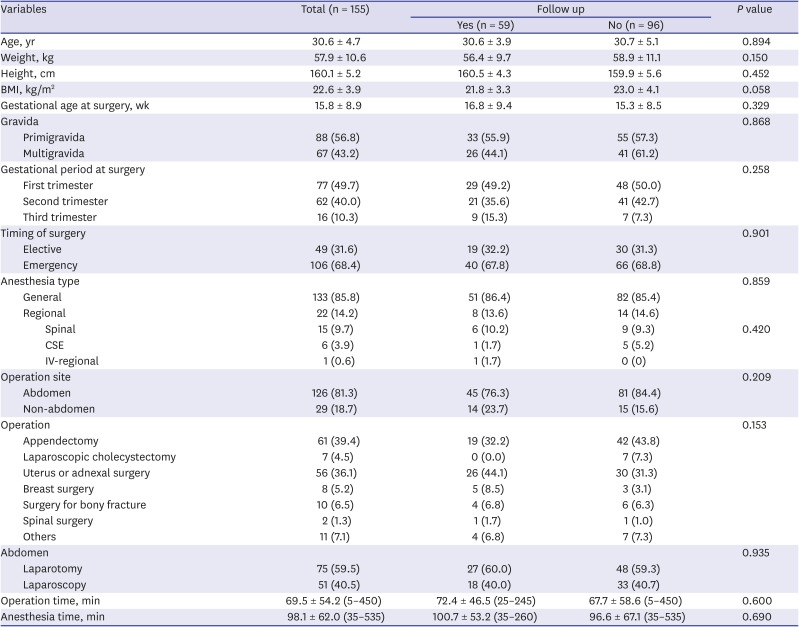
The total number of pregnancy registrations was 16,197 over the 17 years considered. Of these, 155 (0.96%) pregnant women had received non-obstetric surgery under general or regional anesthesia. The demographic characteristics of patients are shown in
Table 1. The mean gestational age at surgery was 15.8 ± 8.9 weeks, and the most common trimester was the first trimester (49.7%). Emergency operations were more than elective operations (68.4% vs. 31.6%). Mean operation time was 69.5 ± 54.2 minutes, and mean anesthesia time was 98.1 ± 62.0 minutes. Most of the patients received general anesthesia (85.8%). The abdomen was the most frequent surgical site, accounting for 81.3% of all sites. Most surgeries were carried out by two departments: general surgery and gynecology (53.5% and 36.1%, respectively), and appendectomy and uterine and adnexal surgery were the two most frequent operations (39.4% and 36.1%, respectively). There was one maternal death two days after the surgery for metastatic brain tumor of breast cancer. The fetus of this deceased woman was at 12 weeks of gestational age and sacrificed when the mother expired. Among these 155 patients, only 59 finished their antenatal care at our hospital, and the others continued their follow-up at another hospital. When comparing the two groups of patients who were classified as completing their follow-up at our hospital or not, the demographic data were not significantly different (
Table 1). The further analyses have proceeded in these 59 patients.
Table 1
Demographic and operational data
|
Variables |
Total (n = 155) |
Follow up |
P value |
|
Yes (n = 59) |
No (n = 96) |
|
Age, yr |
30.6 ± 4.7 |
30.6 ± 3.9 |
30.7 ± 5.1 |
0.894 |
|
Weight, kg |
57.9 ± 10.6 |
56.4 ± 9.7 |
58.9 ± 11.1 |
0.150 |
|
Height, cm |
160.1 ± 5.2 |
160.5 ± 4.3 |
159.9 ± 5.6 |
0.452 |
|
BMI, kg/m2
|
22.6 ± 3.9 |
21.8 ± 3.3 |
23.0 ± 4.1 |
0.058 |
|
Gestational age at surgery, wk |
15.8 ± 8.9 |
16.8 ± 9.4 |
15.3 ± 8.5 |
0.329 |
|
Gravida |
|
|
|
0.868 |
|
Primigravida |
88 (56.8) |
33 (55.9) |
55 (57.3) |
|
Multigravida |
67 (43.2) |
26 (44.1) |
41 (61.2) |
|
Gestational period at surgery |
|
|
|
0.258 |
|
First trimester |
77 (49.7) |
29 (49.2) |
48 (50.0) |
|
Second trimester |
62 (40.0) |
21 (35.6) |
41 (42.7) |
|
Third trimester |
16 (10.3) |
9 (15.3) |
7 (7.3) |
|
Timing of surgery |
|
|
|
0.901 |
|
Elective |
49 (31.6) |
19 (32.2) |
30 (31.3) |
|
Emergency |
106 (68.4) |
40 (67.8) |
66 (68.8) |
|
Anesthesia type |
|
|
|
0.859 |
|
General |
133 (85.8) |
51 (86.4) |
82 (85.4) |
|
Regional |
22 (14.2) |
8 (13.6) |
14 (14.6) |
|
|
Spinal |
15 (9.7) |
6 (10.2) |
9 (9.3) |
0.420 |
|
|
CSE |
6 (3.9) |
1 (1.7) |
5 (5.2) |
|
|
IV-regional |
1 (0.6) |
1 (1.7) |
0 (0) |
|
Operation site |
|
|
|
0.209 |
|
Abdomen |
126 (81.3) |
45 (76.3) |
81 (84.4) |
|
Non-abdomen |
29 (18.7) |
14 (23.7) |
15 (15.6) |
|
Operation |
|
|
|
0.153 |
|
Appendectomy |
61 (39.4) |
19 (32.2) |
42 (43.8) |
|
Laparoscopic cholecystectomy |
7 (4.5) |
0 (0.0) |
7 (7.3) |
|
Uterus or adnexal surgery |
56 (36.1) |
26 (44.1) |
30 (31.3) |
|
Breast surgery |
8 (5.2) |
5 (8.5) |
3 (3.1) |
|
Surgery for bony fracture |
10 (6.5) |
4 (6.8) |
6 (6.3) |
|
Spinal surgery |
2 (1.3) |
1 (1.7) |
1 (1.0) |
|
Others |
11 (7.1) |
4 (6.8) |
7 (7.3) |
|
Abdomen |
|
|
|
0.935 |
|
Laparotomy |
75 (59.5) |
27 (60.0) |
48 (59.3) |
|
Laparoscopy |
51 (40.5) |
18 (40.0) |
33 (40.7) |
|
Operation time, min |
69.5 ± 54.2 (5–450) |
72.4 ± 46.5 (25–245) |
67.7 ± 58.6 (5–450) |
0.600 |
|
Anesthesia time, min |
98.1 ± 62.0 (35–535) |
100.7 ± 53.2 (35–260) |
96.6 ± 67.1 (35–535) |
0.690 |

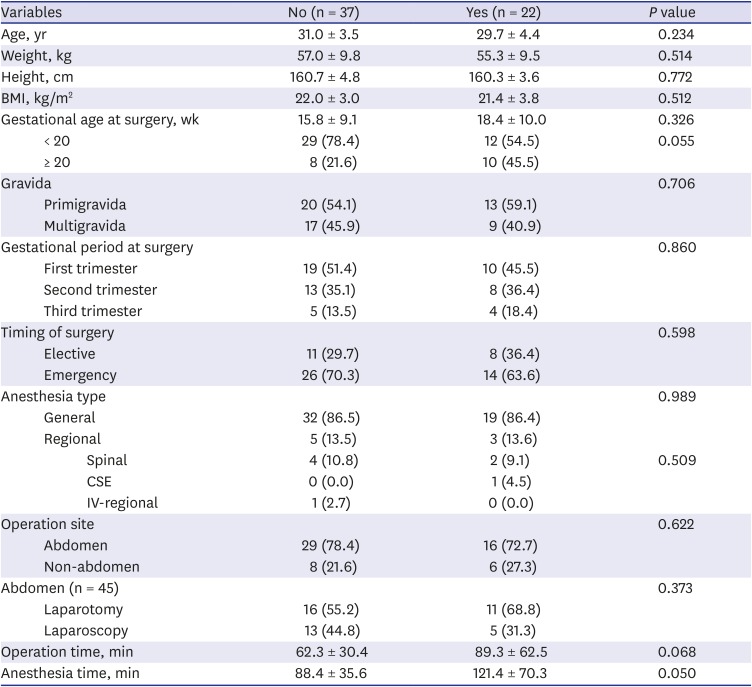
Twenty-two patients conformed to the criteria of the overall adverse outcome. There were six spontaneous abortions, one stillbirth, and seven premature births. The stillbirth case received the surgery at gestational age 18 weeks and lost her fetus at gestational age over 20 weeks. Eight patients experienced preterm labor or threatened abortion but delivered their baby at term. There was no congenital disability. Comparison between the two groups divided by the presence of an overall adverse outcome event is shown in
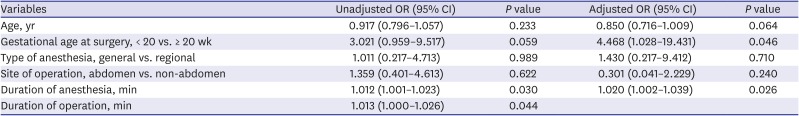
Table 2. Based on this data, multiple logistic regression test included five factors: maternal age (years), gestational age (≥ 20 weeks or < 20 weeks), type of anesthesia (general or regional), operation site (abdominal or non-abdominal), and duration of anesthesia (minutes). Duration of operation was excluded because it had multicollinearity with the duration of anesthesia. Type of anesthesia and operation site was included because these were associated with a higher risk of adverse outcome.
78 The model showed that gestational age and duration of anesthesia were significantly associated with overall adverse outcome (
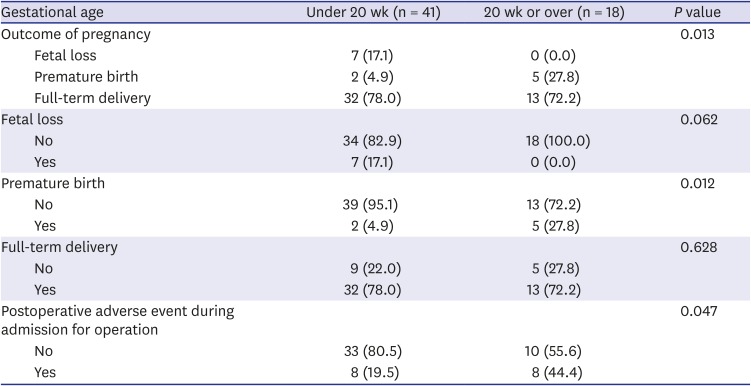
Table 3). Twenty weeks or more gestational age increased the risk 4.5 fold than gestational week less than 20 weeks. Also, whenever the anesthesia time increased by 1 minute, the risk of overall adverse outcome was increased by 2%. The details of adverse events expressed by the dichotomy of patients by 20 weeks of gestational age were shown in
Table 4. The majority of events that occurred in patients under gestational age 20 weeks was fetal loss while all of the adverse events in the patients with gestational age over 20 weeks were preterm labor and followed premature birth.
Table 2
Demographic and operational data by the presence of adverse outcome
|
Variables |
No (n = 37) |
Yes (n = 22) |
P value |
|
Age, yr |
31.0 ± 3.5 |
29.7 ± 4.4 |
0.234 |
|
Weight, kg |
57.0 ± 9.8 |
55.3 ± 9.5 |
0.514 |
|
Height, cm |
160.7 ± 4.8 |
160.3 ± 3.6 |
0.772 |
|
BMI, kg/m2
|
22.0 ± 3.0 |
21.4 ± 3.8 |
0.512 |
|
Gestational age at surgery, wk |
15.8 ± 9.1 |
18.4 ± 10.0 |
0.326 |
|
< 20 |
29 (78.4) |
12 (54.5) |
0.055 |
|
≥ 20 |
8 (21.6) |
10 (45.5) |
|
Gravida |
|
|
0.706 |
|
Primigravida |
20 (54.1) |
13 (59.1) |
|
Multigravida |
17 (45.9) |
9 (40.9) |
|
Gestational period at surgery |
|
|
0.860 |
|
First trimester |
19 (51.4) |
10 (45.5) |
|
Second trimester |
13 (35.1) |
8 (36.4) |
|
Third trimester |
5 (13.5) |
4 (18.4) |
|
Timing of surgery |
|
|
0.598 |
|
Elective |
11 (29.7) |
8 (36.4) |
|
Emergency |
26 (70.3) |
14 (63.6) |
|
Anesthesia type |
|
|
0.989 |
|
General |
32 (86.5) |
19 (86.4) |
|
Regional |
5 (13.5) |
3 (13.6) |
|
|
Spinal |
4 (10.8) |
2 (9.1) |
0.509 |
|
|
CSE |
0 (0.0) |
1 (4.5) |
|
|
IV-regional |
1 (2.7) |
0 (0.0) |
|
Operation site |
|
|
0.622 |
|
Abdomen |
29 (78.4) |
16 (72.7) |
|
Non-abdomen |
8 (21.6) |
6 (27.3) |
|
Abdomen (n = 45) |
|
|
0.373 |
|
Laparotomy |
16 (55.2) |
11 (68.8) |
|
Laparoscopy |
13 (44.8) |
5 (31.3) |
|
Operation time, min |
62.3 ± 30.4 |
89.3 ± 62.5 |
0.068 |
|
Anesthesia time, min |
88.4 ± 35.6 |
121.4 ± 70.3 |
0.050 |

Table 3
Multiple logistic regression analysis to determine the factors that affected adverse events
|
Variables |
Unadjusted OR (95% CI) |
P value |
Adjusted OR (95% CI) |
P value |
|
Age, yr |
0.917 (0.796–1.057) |
0.233 |
0.850 (0.716–1.009) |
0.064 |
|
Gestational age at surgery, < 20 vs. ≥ 20 wk |
3.021 (0.959–9.517) |
0.059 |
4.468 (1.028–19.431) |
0.046 |
|
Type of anesthesia, general vs. regional |
1.011 (0.217–4.713) |
0.989 |
1.430 (0.217–9.412) |
0.710 |
|
Site of operation, abdomen vs. non-abdomen |
1.359 (0.401–4.613) |
0.622 |
0.301 (0.041–2.229) |
0.240 |
|
Duration of anesthesia, min |
1.012 (1.001–1.023) |
0.030 |
1.020 (1.002–1.039) |
0.026 |
|
Duration of operation, min |
1.013 (1.000–1.026) |
0.044 |
|
|

Table 4
Details of adverse events by the dichotomy of patients by 20 weeks of gestational age at the surgery
|
Gestational age |
Under 20 wk (n = 41) |
20 wk or over (n = 18) |
P value |
|
Outcome of pregnancy |
|
|
0.013 |
|
Fetal loss |
7 (17.1) |
0 (0.0) |
|
Premature birth |
2 (4.9) |
5 (27.8) |
|
Full-term delivery |
32 (78.0) |
13 (72.2) |
|
Fetal loss |
|
|
0.062 |
|
No |
34 (82.9) |
18 (100.0) |
|
Yes |
7 (17.1) |
0 (0.0) |
|
Premature birth |
|
|
0.012 |
|
No |
39 (95.1) |
13 (72.2) |
|
Yes |
2 (4.9) |
5 (27.8) |
|
Full-term delivery |
|
|
0.628 |
|
No |
9 (22.0) |
5 (27.8) |
|
Yes |
32 (78.0) |
13 (72.2) |
|
Postoperative adverse event during admission for operation |
|
|
0.047 |
|
No |
33 (80.5) |
10 (55.6) |
|
Yes |
8 (19.5) |
8 (44.4) |

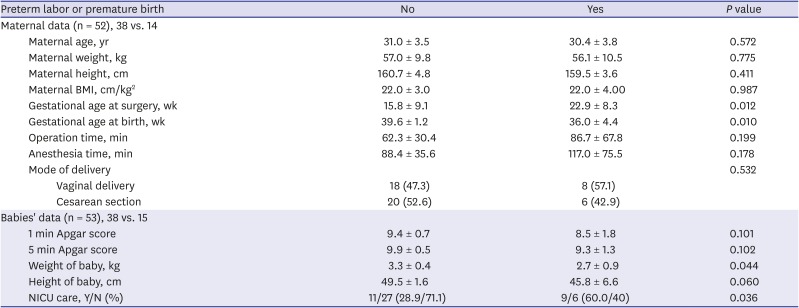
Since there were six spontaneous abortions and one stillbirth, a total of 53 babies were born from 52 mothers, including a couple of twin babies. Maternal variables were analyzed using 52 mothers' data, and neonatal variables included 53 babies' data (
Table 5). Mode of delivery was not significantly different by the presence of preterm labor. Gestational age at surgery was significantly older in the babies of the mothers who experienced adverse outcome event (
P = 0.012). The weight of neonates was significantly lower in the babies from the mothers who experienced adverse outcome event (
P = 0.044). Although the Apgar scores were not significantly lower in these neonates, they were more likely to be admitted into a neonatal intensive care unit (NICU) (
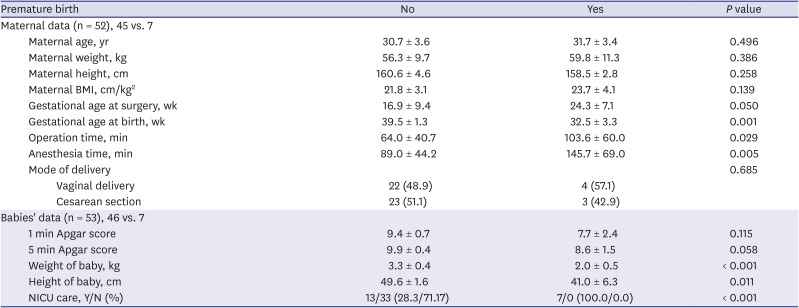
P = 0.036). When these analyses were repeated regarding premature birth, seven neonates were premature, and 46 neonates were delivered at term (
Table 6). Duration of operation and anesthesia were significantly longer in the premature birth group (
P = 0.029 and
P = 0.005, respectively). The differences in the Apgar scores were not significant, but the premature babies showed lower birth weight and height (
P < 0.001 and
P = 0.010, respectively) and were more likely to be admitted into the NICU (
P < 0.001).
Table 5
Maternal and babies’ data by the presence of preterm labor during pregnancy or premature birth
|
Preterm labor or premature birth |
No |
Yes |
P value |
|
Maternal data (n = 52), 38 vs. 14 |
|
|
|
|
Maternal age, yr |
31.0 ± 3.5 |
30.4 ± 3.8 |
0.572 |
|
Maternal weight, kg |
57.0 ± 9.8 |
56.1 ± 10.5 |
0.775 |
|
Maternal height, cm |
160.7 ± 4.8 |
159.5 ± 3.6 |
0.411 |
|
Maternal BMI, cm/kg2
|
22.0 ± 3.0 |
22.0 ± 4.00 |
0.987 |
|
Gestational age at surgery, wk |
15.8 ± 9.1 |
22.9 ± 8.3 |
0.012 |
|
Gestational age at birth, wk |
39.6 ± 1.2 |
36.0 ± 4.4 |
0.010 |
|
Operation time, min |
62.3 ± 30.4 |
86.7 ± 67.8 |
0.199 |
|
Anesthesia time, min |
88.4 ± 35.6 |
117.0 ± 75.5 |
0.178 |
|
Mode of delivery |
|
|
0.532 |
|
|
Vaginal delivery |
18 (47.3) |
8 (57.1) |
|
|
Cesarean section |
20 (52.6) |
6 (42.9) |
|
Babies' data (n = 53), 38 vs. 15 |
|
|
|
|
1 min Apgar score |
9.4 ± 0.7 |
8.5 ± 1.8 |
0.101 |
|
5 min Apgar score |
9.9 ± 0.5 |
9.3 ± 1.3 |
0.102 |
|
Weight of baby, kg |
3.3 ± 0.4 |
2.7 ± 0.9 |
0.044 |
|
Height of baby, cm |
49.5 ± 1.6 |
45.8 ± 6.6 |
0.060 |
|
NICU care, Y/N (%) |
11/27 (28.9/71.1) |
9/6 (60.0/40) |
0.036 |

Table 6
Maternal and babies' data by the presence of premature birth
|
Premature birth |
No |
Yes |
P value |
|
Maternal data (n = 52), 45 vs. 7 |
|
|
|
|
Maternal age, yr |
30.7 ± 3.6 |
31.7 ± 3.4 |
0.496 |
|
Maternal weight, kg |
56.3 ± 9.7 |
59.8 ± 11.3 |
0.386 |
|
Maternal height, cm |
160.6 ± 4.6 |
158.5 ± 2.8 |
0.258 |
|
Maternal BMI, cm/kg2
|
21.8 ± 3.1 |
23.7 ± 4.1 |
0.139 |
|
Gestational age at surgery, wk |
16.9 ± 9.4 |
24.3 ± 7.1 |
0.050 |
|
Gestational age at birth, wk |
39.5 ± 1.3 |
32.5 ± 3.3 |
0.001 |
|
Operation time, min |
64.0 ± 40.7 |
103.6 ± 60.0 |
0.029 |
|
Anesthesia time, min |
89.0 ± 44.2 |
145.7 ± 69.0 |
0.005 |
|
Mode of delivery |
|
|
0.685 |
|
|
Vaginal delivery |
22 (48.9) |
4 (57.1) |
|
|
Cesarean section |
23 (51.1) |
3 (42.9) |
|
Babies' data (n = 53), 46 vs. 7 |
|
|
|
|
1 min Apgar score |
9.4 ± 0.7 |
7.7 ± 2.4 |
0.115 |
|
5 min Apgar score |
9.9 ± 0.4 |
8.6 ± 1.5 |
0.058 |
|
Weight of baby, kg |
3.3 ± 0.4 |
2.0 ± 0.5 |
< 0.001 |
|
Height of baby, cm |
49.6 ± 1.6 |
41.0 ± 6.3 |
0.011 |
|
NICU care, Y/N (%) |
13/33 (28.3/71.17) |
7/0 (100.0/0.0) |
< 0.001 |

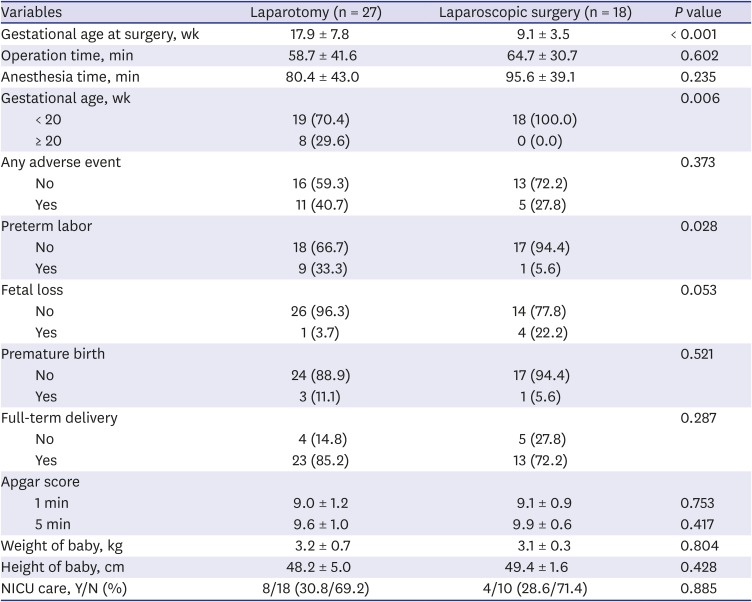
For the surgeries involving the abdomen, the comparison between the patients who had either laparotomy (n = 27) or laparoscopic surgery (n = 18) was performed (
Table 7). Duration of operation and anesthesia were not significantly different in the two groups. Mean gestational age at surgery was significantly older in the laparotomy group (
P < 0.001). All patients at 20 weeks or more of gestation received laparotomy, and there was a difference in choice of surgical method by a datum line gestational age 20 weeks (
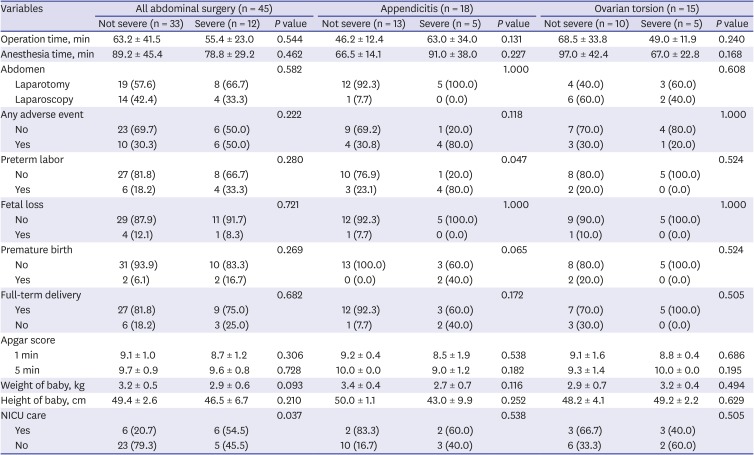
P = 0.006). While the total incidence of overall adverse outcome was not different, the frequency of preterm labor was higher in the laparotomy group. Apgar scores, babies’ weight, and height, and the rate of admission to NICU were not different. In addition, the patients who received abdominal surgery were divided into two severity grades—not severe and severe (
Table 8). We defined a patient as a severe case when there was an abscess formation, peritonitis, gangrenous change, perforation, or hemorrhagic necrosis on the pathologic finding of the specimen. There were no significant differences in terms of operation type (laparotomy or laparoscopy), operation time, anesthesia time, and babies' Apgar scores, height, and weight. Also, there were no differences in the incidence of overall adverse outcome, fetal loss, preterm labor, and premature birth. However, the NICU admission rate was higher in the severe group (
P = 0.037). When the subgroup analyses were performed, the incidence of preterm labor was higher in severe appendicitis group (
P = 0.047), while other variables were not significantly different.
Table 7
Data of abdominal surgery by type of operation
|
Variables |
Laparotomy (n = 27) |
Laparoscopic surgery (n = 18) |
P value |
|
Gestational age at surgery, wk |
17.9 ± 7.8 |
9.1 ± 3.5 |
< 0.001 |
|
Operation time, min |
58.7 ± 41.6 |
64.7 ± 30.7 |
0.602 |
|
Anesthesia time, min |
80.4 ± 43.0 |
95.6 ± 39.1 |
0.235 |
|
Gestational age, wk |
|
|
0.006 |
|
< 20 |
19 (70.4) |
18 (100.0) |
|
≥ 20 |
8 (29.6) |
0 (0.0) |
|
Any adverse event |
|
|
0.373 |
|
No |
16 (59.3) |
13 (72.2) |
|
Yes |
11 (40.7) |
5 (27.8) |
|
Preterm labor |
|
|
0.028 |
|
No |
18 (66.7) |
17 (94.4) |
|
Yes |
9 (33.3) |
1 (5.6) |
|
Fetal loss |
|
|
0.053 |
|
No |
26 (96.3) |
14 (77.8) |
|
Yes |
1 (3.7) |
4 (22.2) |
|
Premature birth |
|
|
0.521 |
|
No |
24 (88.9) |
17 (94.4) |
|
Yes |
3 (11.1) |
1 (5.6) |
|
Full-term delivery |
|
|
0.287 |
|
No |
4 (14.8) |
5 (27.8) |
|
Yes |
23 (85.2) |
13 (72.2) |
|
Apgar score |
|
|
|
|
1 min |
9.0 ± 1.2 |
9.1 ± 0.9 |
0.753 |
|
5 min |
9.6 ± 1.0 |
9.9 ± 0.6 |
0.417 |
|
Weight of baby, kg |
3.2 ± 0.7 |
3.1 ± 0.3 |
0.804 |
|
Height of baby, cm |
48.2 ± 5.0 |
49.4 ± 1.6 |
0.428 |
|
NICU care, Y/N (%) |
8/18 (30.8/69.2) |
4/10 (28.6/71.4) |
0.885 |

Table 8
Data of abdominal surgery by disease severity
|
Variables |
All abdominal surgery (n = 45) |
Appendicitis (n = 18) |
Ovarian torsion (n = 15) |
|
Not severe (n = 33) |
Severe (n = 12) |
P value |
Not severe (n = 13) |
Severe (n = 5) |
P value |
Not severe (n = 10) |
Severe (n = 5) |
P value |
|
Operation time, min |
63.2 ± 41.5 |
55.4 ± 23.0 |
0.544 |
46.2 ± 12.4 |
63.0 ± 34.0 |
0.131 |
68.5 ± 33.8 |
49.0 ± 11.9 |
0.240 |
|
Anesthesia time, min |
89.2 ± 45.4 |
78.8 ± 29.2 |
0.462 |
66.5 ± 14.1 |
91.0 ± 38.0 |
0.227 |
97.0 ± 42.4 |
67.0 ± 22.8 |
0.168 |
|
Abdomen |
|
|
0.582 |
|
|
1.000 |
|
|
0.608 |
|
Laparotomy |
19 (57.6) |
8 (66.7) |
12 (92.3) |
5 (100.0) |
4 (40.0) |
3 (60.0) |
|
Laparoscopy |
14 (42.4) |
4 (33.3) |
1 (7.7) |
0 (0.0) |
6 (60.0) |
2 (40.0) |
|
Any adverse event |
|
|
0.222 |
|
|
0.118 |
|
|
1.000 |
|
No |
23 (69.7) |
6 (50.0) |
9 (69.2) |
1 (20.0) |
7 (70.0) |
4 (80.0) |
|
Yes |
10 (30.3) |
6 (50.0) |
4 (30.8) |
4 (80.0) |
3 (30.0) |
1 (20.0) |
|
Preterm labor |
|
|
0.280 |
|
|
0.047 |
|
|
0.524 |
|
No |
27 (81.8) |
8 (66.7) |
10 (76.9) |
1 (20.0) |
8 (80.0) |
5 (100.0) |
|
Yes |
6 (18.2) |
4 (33.3) |
3 (23.1) |
4 (80.0) |
2 (20.0) |
0 (0.0) |
|
Fetal loss |
|
|
0.721 |
|
|
1.000 |
|
|
1.000 |
|
No |
29 (87.9) |
11 (91.7) |
12 (92.3) |
5 (100.0) |
9 (90.0) |
5 (100.0) |
|
Yes |
4 (12.1) |
1 (8.3) |
1 (7.7) |
0 (0.0) |
1 (10.0) |
0 (0.0) |
|
Premature birth |
|
|
0.269 |
|
|
0.065 |
|
|
0.524 |
|
No |
31 (93.9) |
10 (83.3) |
13 (100.0) |
3 (60.0) |
8 (80.0) |
5 (100.0) |
|
Yes |
2 (6.1) |
2 (16.7) |
0 (0.0) |
2 (40.0) |
2 (20.0) |
0 (0.0) |
|
Full-term delivery |
|
|
0.682 |
|
|
0.172 |
|
|
0.505 |
|
Yes |
27 (81.8) |
9 (75.0) |
12 (92.3) |
3 (60.0) |
7 (70.0) |
5 (100.0) |
|
No |
6 (18.2) |
3 (25.0) |
1 (7.7) |
2 (40.0) |
3 (30.0) |
0 (0.0) |
|
Apgar score |
|
|
|
|
|
|
|
|
|
|
1 min |
9.1 ± 1.0 |
8.7 ± 1.2 |
0.306 |
9.2 ± 0.4 |
8.5 ± 1.9 |
0.538 |
9.1 ± 1.6 |
8.8 ± 0.4 |
0.686 |
|
5 min |
9.7 ± 0.9 |
9.6 ± 0.8 |
0.728 |
10.0 ± 0.0 |
9.0 ± 1.2 |
0.182 |
9.3 ± 1.4 |
10.0 ± 0.0 |
0.195 |
|
Weight of baby, kg |
3.2 ± 0.5 |
2.9 ± 0.6 |
0.093 |
3.4 ± 0.4 |
2.7 ± 0.7 |
0.116 |
2.9 ± 0.7 |
3.2 ± 0.4 |
0.494 |
|
Height of baby, cm |
49.4 ± 2.6 |
46.5 ± 6.7 |
0.210 |
50.0 ± 1.1 |
43.0 ± 9.9 |
0.252 |
48.2 ± 4.1 |
49.2 ± 2.2 |
0.629 |
|
NICU care |
|
|
0.037 |
|
|
0.538 |
|
|
0.505 |
|
Yes |
6 (20.7) |
6 (54.5) |
2 (83.3) |
2 (60.0) |
3 (66.7) |
3 (40.0) |
|
No |
23 (79.3) |
5 (45.5) |
10 (16.7) |
3 (40.0) |
6 (33.3) |
2 (60.0) |

Go to :

DISCUSSION
The incidence of non-obstetric surgery during pregnancy was calculated as 0.96% in this study. It did not deviate much from the results of previous studies.
1234 The main results of our retrospective analyses were the following. First, a non-obstetric surgery during pregnancy performed at over 20 weeks of gestational age was associated with a higher incidence of an adverse event than that of the surgery performed before 20 weeks in perspective of total adverse outcome including preterm labor, premature birth, and fetal loss. Second, prolonged operation and anesthesia time related to the higher adverse outcome.
Approximately 50% of surgery was performed in the first trimester. Mazze et al.
13 reported the distribution of surgery by trimester as 42% in the first trimester, 35% in the second trimester, and 23% in the third trimester. Guidelines recommend performing required surgeries in the second trimester in possible cases to avoid the critical period of organogenesis in early pregnancy and enlarged gravid uterus in late pregnancy, which can lead to surgical technical difficulties and difficult airway of the mother.
121415 However, these guidelines commonly emphasized that surgery should never be denied or delayed in the indicated situation, regardless of trimester. In this study, there was no difference in the aspect of the overall adverse outcome among the trimesters, but the logistic regression analysis showed an increased risk in patients in the second half of gestation. Similarly, Ibiebele et al.
10 recommended that preparing for managing preterm labor should be accompanied when an appendectomy is performed in a pregnant woman over 20 weeks of gestational age.
At first glance, our results indicate that the operational type of abdominal surgery affected the incidence of preterm labor. Similarly, a recent systematic review and meta-analysis, and a recent large scale research study found an increased risk of miscarriage in laparoscopic appendectomy patients than in open appendectomy patients.
19 However, there was a lack of description about gestational age at the surgery in both studies. Because the gestational age was the significant factor in the decision between laparotomy and laparoscopic surgery due to a technical difficulty, we cannot conclude yet about which factor—gestational age or surgical method—affects the different occurrence of adverse events and which method would take a superior position in abdominal surgery of pregnant women. Laparoscopic surgery was contraindicated in pregnant women in the past, but this is no longer true.
61617 However, laparoscopic surgery in pregnant women is still controversial. The main disadvantage is associated with the pneumoperitoneum produced by carbon dioxide, which increases the risk for hypoxemia and hypercapnia, which can lead to fetal acidosis or asphyxia.
18 In addition, the enlarged uterus increases the risk of damage by trocar.
1619 In a meta-analysis of 11 studies, Wilasrusmee et al.
9 reported that the incidence of fetal loss was significantly higher in a laparoscopic group than in a laparotomy group, with a relative risk of 1.91. There is a need for a controlled comparison between laparoscopic surgery and laparotomy in patients with similar gestational age.
In appendectomy during pregnancy, it is known that disease severity is related to maternal and fetal outcomes.
2021 Walsh et al.
20 reported 12.1% of fetal loss rate in complicated appendicitis with perforation, abscess, or peritonitis, while 3.4% in simple appendicitis. From our results, we also observed the higher rate of preterm labor with severe appendicitis. Also, the NICU admission rate was higher in severe cases when we included elective abdominal surgeries and ovarian torsion cases in the analysis (
Table 8). Therefore, avoidance of delaying diagnosis and decision for treatment is important,
21 and close observation for the occurrence of preterm labor would be necessary.
There was a difference in the pattern of operation and anesthesia time between appendicitis and ovarian torsion. In the appendicitis cases, the operation time was longer in severe cases, while it took less time in the ovarian torsion cases. It might be because the choice of operation could be different by the severity of ovarian torsion. Ovarian torsion with necrotic change would receive salpingo-oophorectomy, while ovarian cystectomy would be performed in a case without necrotic change. The longer operation time in the less severe cases could contribute to unfavorable outcomes and result in the insignificance of the outcome of pregnancy between the severe group and the not severe group. The small number of patients also would contribute to the insignificance.
The incidence of an adverse event after surgery was higher in women whose pregnancy was beyond 20 weeks, but there was no fetal loss in those patients while seven fetal losses occurred in the patients whose pregnancy was less than 20 weeks. Average gestational age of these fetal loss cases was 8.5 weeks, and preterm labor and premature birth were more likely to occur in the second half of pregnancy. Therefore, we have to emphasize a different strategy of postoperative care after non-obstetric surgery: monitoring and avoiding spontaneous abortion in patients in early pregnancy and preparing for and managing preterm labor in patients in the second half of pregnancy.
Regarding the type of anesthesia, there is no clear conclusion about which method is better between general anesthesia and regional anesthesia. Mazze et al.
13 reported that no specific type of anesthesia was associated with higher adverse outcomes. Hong
8 reported that pregnant women who received surgery via laparotomy for adnexal operation under regional anesthesia showed a higher risk of preterm labor than general anesthesia. In our study, there were no significant differences between the two anesthesia types. A recent study by Devroe et al.
11 mentioned that they could not find an independent association between type of anesthesia and outcomes but they recommended that regional anesthesia should be considered whenever possible because of low birth weight and neurotoxicity of anesthetics.
General anesthesia is generally expected to have a negative impact on maternal and fetal outcomes. The impact includes a higher risk of low birth weight of neonates from mothers who underwent surgery during pregnancy.
11322 In the present study, we observed lower birth weight in the neonates of mothers who experienced any adverse events during their pregnancy after surgery than the neonates of mothers who did not experience an adverse event. Therefore, physicians involved in the non-obstetric surgery should make an effort to avoid it as much as possible.
The operation time and the anesthesia time showed correlation with the occurrence of adverse events. Moreover, a strong correlation between premature birth and the duration of operation and anesthesia was observed. Anesthetic time is likely to correspond to operation time. These durations also might be related to the factors such as type of operation, underlying maternal condition necessitating the surgery, and severity of the disease. Taken together, it seems reasonable to choose the more preferred method for the surgeon and the anesthetist to shorten the operation and anesthesia time considering the patient's status at the time of surgery.
There are several limitations to this study. First, it was a retrospective study based on medical records; therefore, there might be confounding factors that were not able to be controlled. Second, because it was a single-center study, the pool of patients was small, and furthermore, only 59 patients finished their antenatal care at our hospital. At a tertiary hospital, it is highly likely to have a high incidence of high-risk pregnancies in general; or pregnancies that are high risk for preterm labor, premature birth, and miscarriage. Therefore, there might be a selection bias. Third, we did not compare women who did not receive any surgery during pregnancy.
In conclusion, adverse condition progression in mothers was associated with gestational age and duration of operation and anesthesia. Therefore, all physicians who participated in the surgery should acknowledge and prepare for common possible adverse events at that stage of pregnancy and cooperate with each other to finish the surgery smoothly without wasting time.
Go to :













 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download