1. Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012; 48(1):82–96. PMID:
22088488.

2. Olsen RG, Ballinger MB, David TD, Lotz WG. Rewarming of the hypothermic rhesus monkey with electromagnetic radiation. Bioelectromagnetics. 1987; 8(2):183–193. PMID:
3619952.

3. Aversi-Ferreira TA, Aversi-Ferreira RA, Bretas RV, Nishimaru H, Nishijo H. Comparative anatomy of the arm muscles of the Japanese monkey (
Macaca fuscata) with some comments on locomotor mechanics and behavior. J Med Primatol. 2016; 45(4):165–179. PMID:
27297259.
4. Decramer T, Swinnen S, van Loon J, Janssen P, Theys T. White matter tract anatomy in the rhesus monkey: a fiber dissection study. Brain Struct Funct. 2018; 223(8):3681–3688. PMID:
30022250.

5. Besle J, Mougin O, Sánchez-Panchuelo RM, Lanting C, Gowland P, Bowtell R, et al. Is human auditory cortex organization compatible with the monkey model? Contrary evidence from ultra-high-field functional and structural MRI. Cereb Cortex. 2019; 29(1):410–428. PMID:
30357410.

6. Schilling KG, Gao Y, Christian M, Janve V, Stepniewska I, Landman BA, et al. A web-based atlas combining MRI and histology of the squirrel monkey brain. Neuroinformatics. 2019; 17(1):131–145. PMID:
30006920.

7. Gao Y, Chen W, Zhang X. Investigating the influence of spatial constraints on ultimate receive coil performance for monkey brain MRI at 7T. IEEE Trans Med Imaging. 2018; 37(7):1723–1732. PMID:
29969422.
8. Park JS, Shin HK, Shin BS, Kwon K. True-color face peeled images with botulinum toxin injection sites and anatomic landmarks. Int J Morphol. 2019; 37(3):1016–1022.

9. Lee SB, Chung BS, Chung MS, Youn C, Park JS. Browsing software of the head sectioned images for the android mobile device. Int J Morphol. 2017; 35(4):1377–1382.

10. Kwon K, Chung MS, Park JS, Shin BS, Chung BS. Improved software to browse the serial medical images for learning. J Korean Med Sci. 2017; 32(7):1195–1201. PMID:
28581279.

11. Kwon K, Shin HK, Shin BS, Park JS. Serially peeled images of the curved surface of the face based on cross-sectional images for use in plastic surgery. J Plast Reconstr Aesthet Surg. 2016; 69(5):727–729.

12. Park HS, Chung MS, Shin DS, Jung YW, Park JS. Whole courses of the oculomotor, trochlear, and abducens nerves, identified in sectioned images and surface models. Anat Rec (Hoboken). 2015; 298(2):436–443. PMID:
25212480.

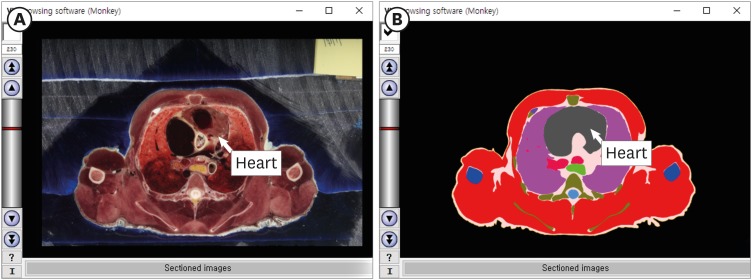
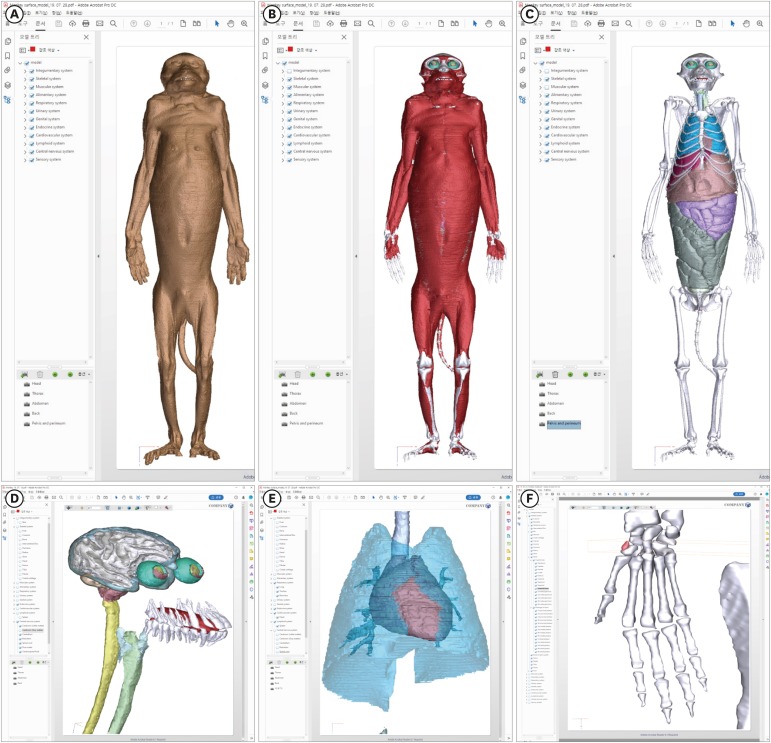
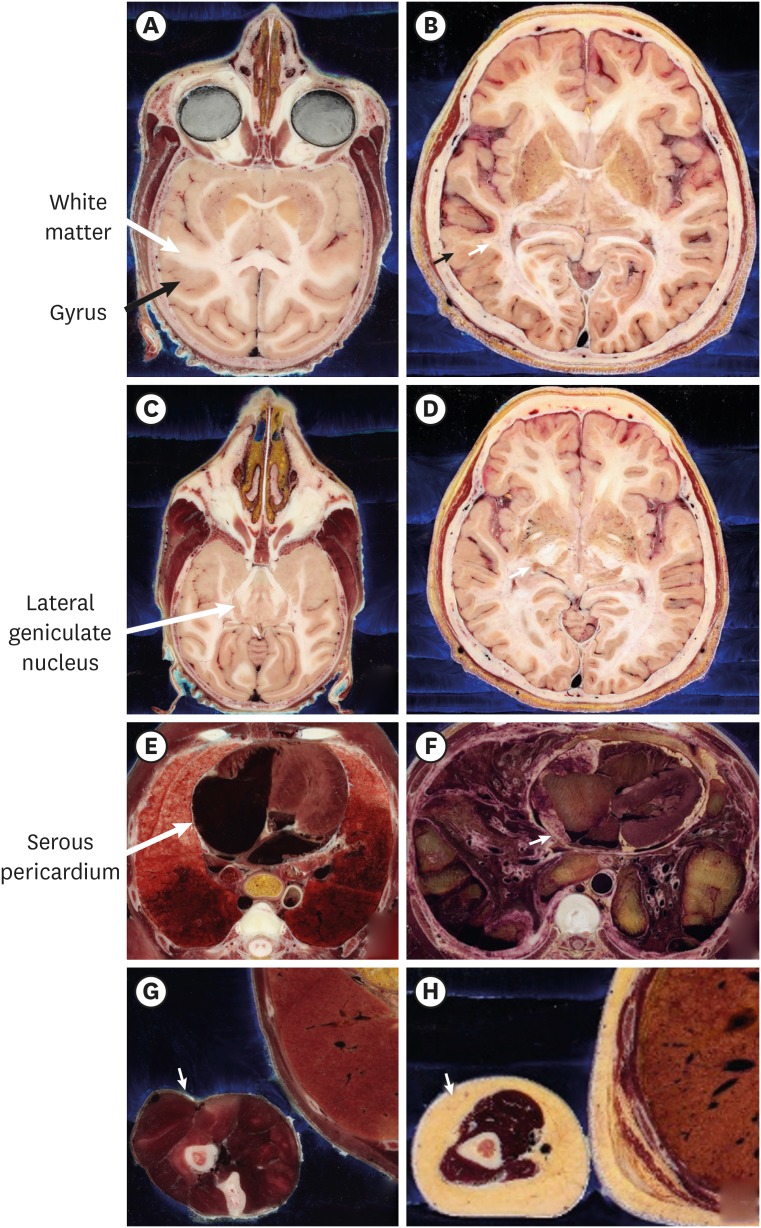
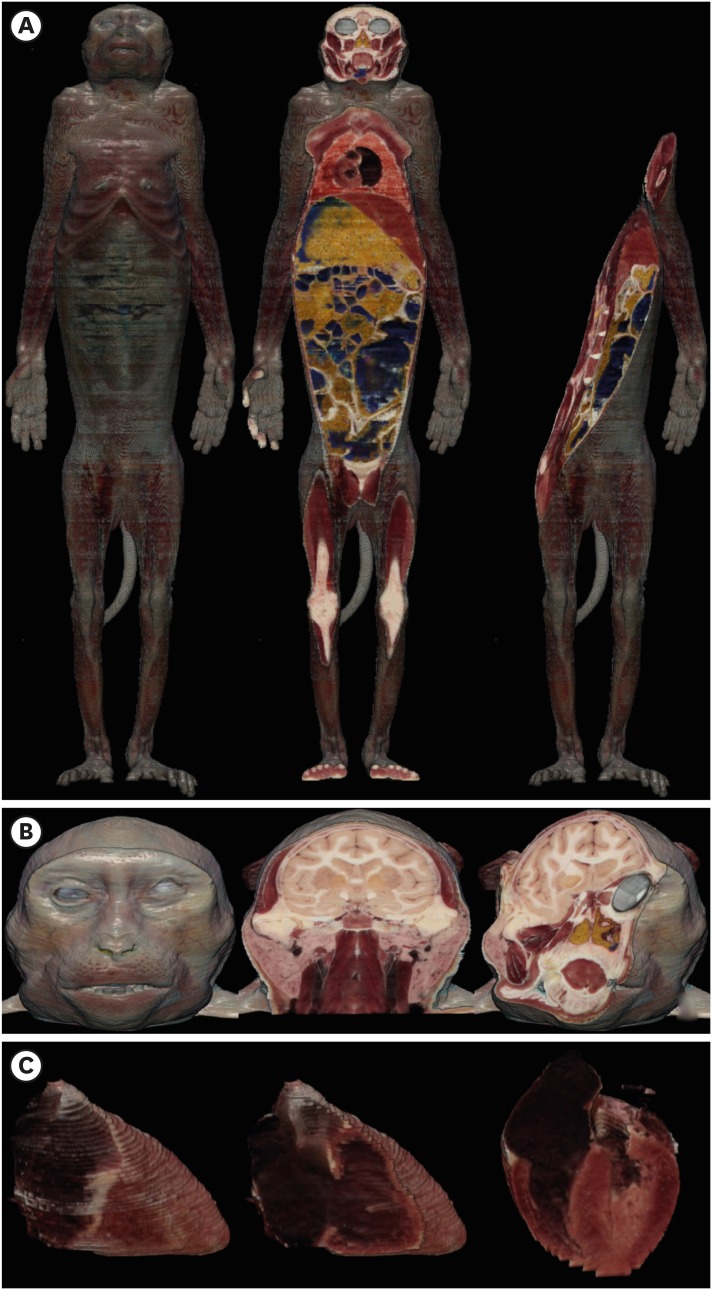
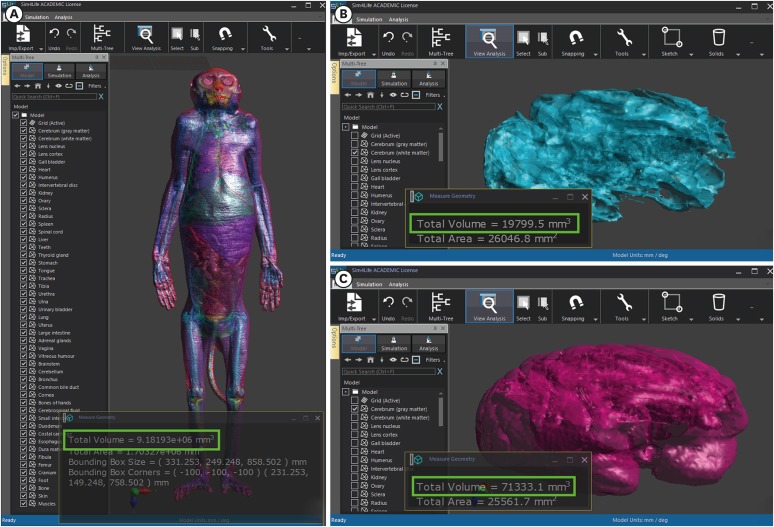
13. Chung BS, Jeon CY, Huh JW, Jeong KJ, Har D, Kwack KS, et al. Rise of the Visible Monkey: sectioned images of rhesus monkey. J Korean Med Sci. 2019; 34(8):e66. PMID:
30833883.

14. Park JS, Chung MS, Hwang SB, Lee YS, Har DH. Technical report on semiautomatic segmentation using the Adobe Photoshop. J Digit Imaging. 2005; 18(4):333–343. PMID:
16003588.

15. Shin DS, Jang HG, Park JS, Park HS, Lee S, Chung MS. Accessible and informative sectioned images and surface models of a cadaver head. J Craniofac Surg. 2012; 23(4):1176–1180. PMID:
22801119.

16. Park JS, Chung MS, Chi JG, Park HS, Shin DS. Segmentation of cerebral gyri in the sectioned images by referring to volume model. J Korean Med Sci. 2010; 25(12):1710–1715. PMID:
21165283.

17. Yeom YS, Jeong JH, Kim CH, Han MC, Ham BK, Cho KW, et al. HDRK-Woman: whole-body voxel model based on high-resolution color slice images of Korean adult female cadaver. Phys Med Biol. 2014; 59(14):3969–3984. PMID:
24971755.

18. Park J, Chung B, Chung M. Digital anatomy using the surface models in portable document format file for self-learning and evaluation. Digit Media. 2017; 3(3):133–137.
19. Chung BS, Park JS. Real-color volume models made from real-color sectioned images of Visible Korean. J Korean Med Sci. 2019; 34(10):e86. PMID:
30886552.

20. Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol. 1996; 41(11):2231–2249. PMID:
8938024.

21. Christ A, Kainz W, Hahn EG, Honegger K, Zefferer M, Neufeld E, et al. The Virtual Family--development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010; 55(2):N23–38. PMID:
20019402.

22. Bragg EM, Fairless EA, Liu S, Briggs F. Morphology of visual sector thalamic reticular neurons in the macaque monkey suggests retinotopically specialized, parallel stream-mixed input to the lateral geniculate nucleus. J Comp Neurol. 2017; 525(5):1273–1290. PMID:
27778378.

23. Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000; 216(3):672–682. PMID:
10966694.

24. Lee AK, Park JS, Hong SE, Taki M, Wake K, Wiart J, et al. Brain SAR of average male Korean child to adult models for mobile phone exposure assessment. Phys Med Biol. 2019; 64(4):045004. PMID:
30719982.

25. Choi C, Nguyen TT, Yeom YS, Lee H, Han H, Shin B, et al. Mesh-type reference Korean phantoms (MRKPs) for adult male and female for use in radiation protection dosimetry. Phys Med Biol. 2019; 64(8):085020. PMID:
30818284.

26. Neufeld E, Lloyd B, Schneider B, Kainz W, Kuster N. Functionalized anatomical models for computational life sciences. Front Physiol. 2018; 9:1594. PMID:
30505279.

27. Park JS, Jung YW, Choi HD, Lee AK. VK-phantom male with 583 structures and female with 459 structures, based on the sectioned images of a male and a female, for computational dosimetry. J Radiat Res (Tokyo). 2018; 59(3):338–380. PMID:
29659988.

28. Werner H, Lopes J, Ribeiro G, Raposo AB, Trajano E, Araujo Júnior E. Three-dimensional virtual traveling navigation and three-dimensional printing models of a normal fetal heart using ultrasonography data. Prenat Diagn. 2019; 39(3):175–177. PMID:
30635939.

29. Tofte JN, Westerlind BO, Martin KD, Guetschow BL, Uribe-Echevarria B, Rungprai C, et al. Knee, Shoulder, and Fundamentals of Arthroscopic Surgery Training: Validation of a Virtual Arthroscopy Simulator. Arthroscopy. 2017; 33(3):641–646.e3. PMID:
27989355.

30. Chung BS, Han M, Har D, Park JS. Advanced sectioned images of a cadaver head with voxel size of 0.04 mm. J Korean Med Sci. 2019; 34(34):e218. PMID:
31456382.

31. Andrews C, Southworth MK, Silva JN, Silva JR. Extended reality in medical practice. Curr Treat Options Cardiovasc Med. 2019; 21(4):18. PMID:
30929093.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download