1. Ezekwudo DE, Ifabiyi T, Gbadamosi B, Haberichter K, Yu Z, Amin M, et al. Breast implant-associated anaplastic large cell lymphoma: a case report and review of the literature. Case Rep Oncol Med. 2017; 2017:6478467. PMID:
29225983.

2. Cordeiro PG, Ghione P, Ni A, Qunying H, Ganesan N, Galasso N, et al. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J Plast Reconstr Aesthet Surg. 2020; DOI:
10.1016/j.bjps.2019.11.064.
3. Cook JA, Sasor SE, Tholpady SS, Chu MW, Momeni A. Complexity of health news reporting on breast implant-associated anaplastic large cell lymphoma. Breast J. 2019; 25(1):163–165. PMID:
30592350.

6. Rohrich RJ. A review of breast-implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2019; 143(3 Suppl):1S–2S.
7. Clemens MW. Current controversies in breast implant–associated anaplastic large cell lymphoma. Aesthet Surg J. 2019; 39(Suppl 1):S1–S2. PMID:
30715175.
8. Deva AK. A perspective on the never-ending cycle of breast implant crises. Aesthet Surg J. 2019; 39(4):NP85–NP86. PMID:
30759184.

9. Swanson E. Textured breast implants, anaplastic large-cell lymphoma, and conflict of interest. Plast Reconstr Surg. 2017; 139(2):558e–559e.

10. Swanson E. Breast implant–associated anaplastic large cell lymphoma (BIA-ALCL): why the search for an infectious etiology may be irrelevant. Aesthet Surg J. 2017; 37(9):NP118–21. PMID:
29025238.

11. Swanson E, Mackay DR. Why the micromort concept falls short in breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) risk analysis. Aesthet Surg J. 2018; 38(3):NP68–NP70. PMID:
29365043.

12. Swanson E, Brown T. A discussion of conflicts of interest in plastic surgery and possible remedies. Plast Reconstr Surg Glob Open. 2018; 6(12):e2043. PMID:
30656120.

13. de Jong D, Vasmel WL, de Boer JP, Verhave G, Barbé E, Casparie MK, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008; 300(17):2030–2035. PMID:
18984890.

14. Srinivasa DR, Miranda RN, Kaura A, Francis AM, Campanale A, Boldrini R, et al. Global adverse event reports of breast implant-associated ALCL: an international review of 40 government authority databases. Plast Reconstr Surg. 2017; 139(5):1029–1039. PMID:
28157770.
15. ElHawary H, Ghazawi N, Efanov J, Izadpanah A. Abstract 31: a systematic review of breast-implant associated anaplastic large cell lymphoma (BIA-ALCL): past and current knowledge. Plast Reconstr Surg Glob Open. 2019; 7(4):Suppl. 22–23.
16. Johnson L, O'Donoghue JM, McLean N, Turton P, Khan AA, Turner SD, et al. Breast implant associated anaplastic large cell lymphoma: The UK experience. Recommendations on its management and implications for informed consent. Eur J Surg Oncol. 2017; 43(8):1393–1401. PMID:
28596034.

17. Campanale A, Boldrini R. BIA-ALCL incidence: the variable to be included in the denominator. Plast Reconstr Surg. 2018; 141(5):779e.
18. de Boer M, van Leeuwen FE, Hauptmann M, Overbeek LI, de Boer JP, Hijmering NJ, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol. 2018; 4(3):335–341. PMID:
29302687.

19. Fitzal F, Turner SD, Kenner L. Is breast implant-associated anaplastic large cell lymphoma a hazard of breast implant surgery? Open Biol. 2019; 9(4):190006. PMID:
30939983.

20. Kricheldorff J, Fallenberg EM, Solbach C, Gerber-Schäfer C, Rancsó C, Fritschen UV. Breast implant-associated lymphoma. Dtsch Arztebl Int. 2018; 115(38):628–635. PMID:
30373708.

21. Doren EL, Miranda RN, Selber JC, Garvey PB, Liu J, Medeiros LJ, et al. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017; 139(5):1042–1050. PMID:
28157769.

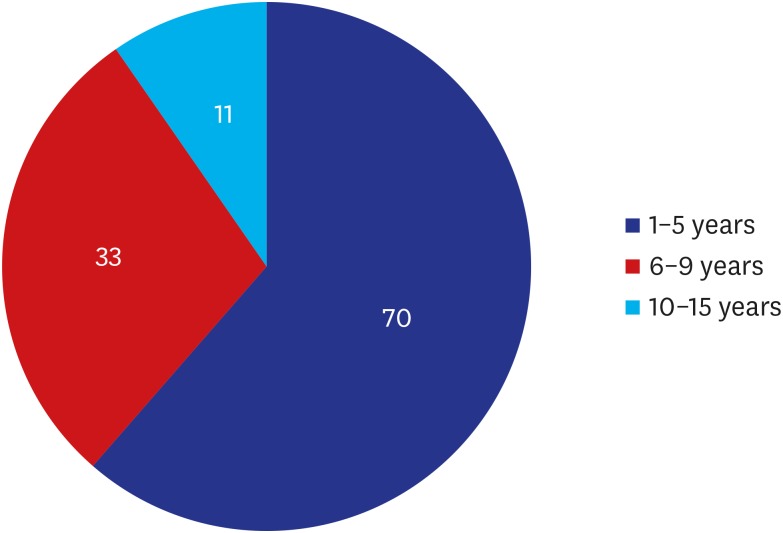
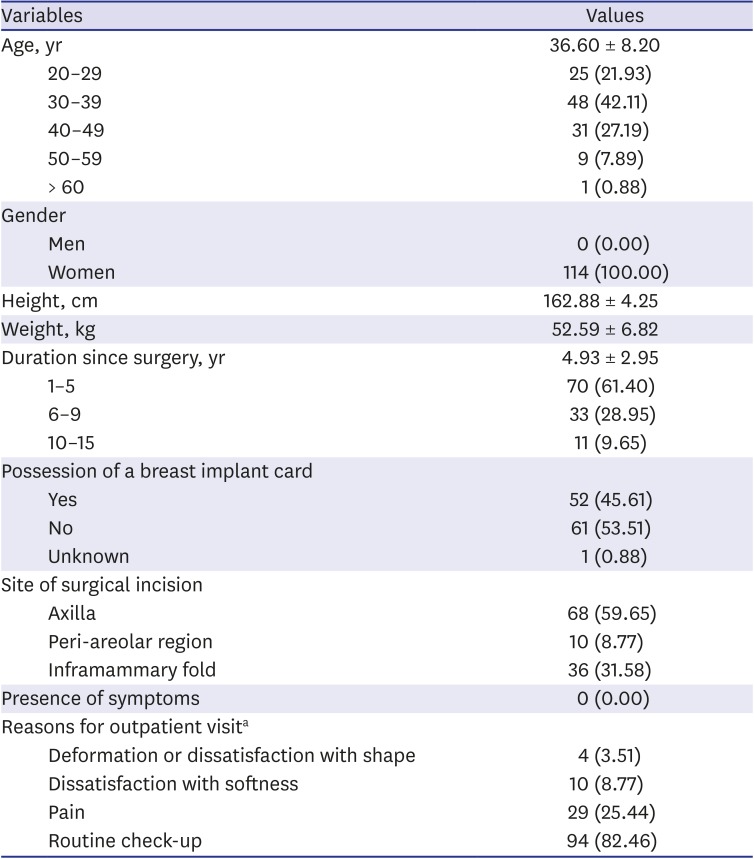
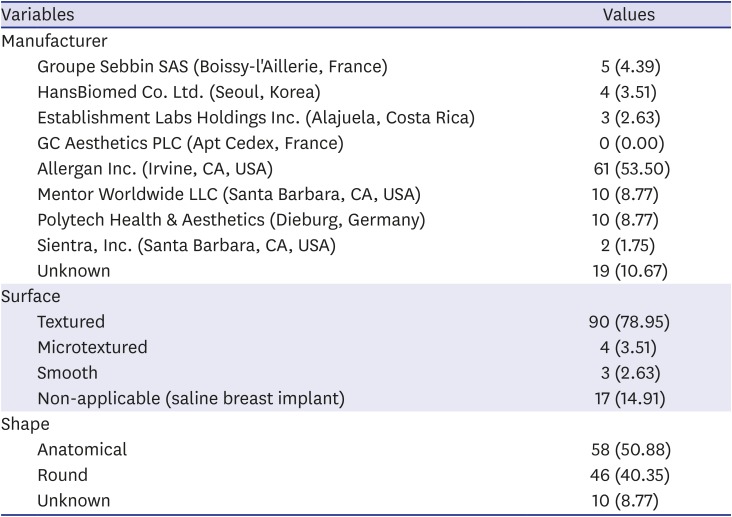
23. “In Korea between 2007 and 2018, women received a total of 222,470 textured breast implants with a risk of breast implant-associated analplastic large cell lymphoma”. Updated 2019. Accessed October 5, 2019.
http://medigatenews.com/news/2630587104.
24. Park AY, Seo BK, Cho KR, Woo OH. The utility of MicroPure™ ultrasound technique in assessing grouped microcalcifications without a mass on mammography. J Breast Cancer. 2016; 19(1):83–86. PMID:
27066098.

27. Han J, Jeong JH, Bang SI, Heo CY. BellaGel breast implant: 4-year results of a prospective cohort study. J Plast Surg Hand Surg. 2019; 53(4):232–239. PMID:
30888239.

28. Sung JY, Jeong JP, Moon DS, Kim MS, Kim HC, Choi WS, et al. Short-term safety of augmentation mammaplasty using the BellaGel implants in Korean women. Plast Reconstr Surg Glob Open. 2019; 7(12):e2566.

29. Sieber DA, Stark RY, Chase S, Schafer M, Adams WP Jr. Clinical evaluation of shaped gel breast implant rotation using high-resolution ultrasound. Aesthet Surg J. 2017; 37(3):290–296. PMID:
28207033.

30. Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. 2011; 127(1):56–66. PMID:
21200201.

31. Brody GS, Deapen D, Taylor CR, Pinter-Brown L, House-Lightner SR, Andersen JS, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015; 135(3):695–705. PMID:
25490535.
32. Hidalgo DA, Weinstein AL. Intraoperative comparison of anatomical versus round implants in breast augmentation: a randomized controlled trial. Plast Reconstr Surg. 2017; 139(3):587–596. PMID:
28234826.
33. Friedman T, Davidovitch N, Scheflan M. Comparative double blind clinical study on round versus shaped cohesive gel implants. Aesthet Surg J. 2006; 26(5):530–536. PMID:
19338941.

34. Gahm J, Edsander-Nord A, Jurell G, Wickman M. No differences in aesthetic outcome or patient satisfaction between anatomically shaped and round expandable implants in bilateral breast reconstructions: a randomized study. Plast Reconstr Surg. 2010; 126(5):1419–1427. PMID:
20639801.

35. Hall-Findlay EJ. Discussion: late seromas and breast implants: theory and practice. Plast Reconstr Surg. 2012; 130(2):436–438. PMID:
22842415.
36. Sforza M, Zaccheddu R, Alleruzzo A, Seno A, Mileto D, Paganelli A, et al. Preliminary 3-year evaluation of experience with SilkSurface and VelvetSurface motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J. 2018; 38(suppl_2):S62–S73. PMID:
29040364.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download