INTRODUCTION
Resistant hypertension is responsible for a considerable proportion of cardiovascular disease events; however, previous treatment strategies based on pharmacotherapy have been unsuccessful in blood pressure (BP) control.
1)2) In the last decade, a novel treatment strategy based on renal sympathetic denervation (RDN) with a percutaneous ablation catheter was introduced. Early studies including the Symplicity-1 and Symplicity-2 trials, which were proof-of-concept and open-label, randomized trials, respectively, showed that catheter-based RDN significantly reduce BP in resistant hypertension.
3)4) Global SYMPLICITY Registry (GSR) and GSR Korea also showed that RDN resulted in significant reduction in office and ambulatory blood pressure both in Caucasian and Asian population.
5)6) However, the Symplicity-3 trial, which compared renal denervation with a sham procedure, failed to show significant BP reduction in resistant hypertension.
7) Post-hoc analysis of Symplicity-3 indicated that insufficient ablation numbers in both renal arteries and failure to complete quadrant ablation may have contributed to the negative results of the study.
8)9) This led to the development of novel renal denervation devices, which increase the number of ablations or ensure the delivery of radiofrequency (RF) ablation, or new energy-based modalities such as ultrasound or cryoablation.
10)11)12)13)
The DENEX™ system is a novel percutaneous RDN system that adopts a basket-type, folding/unfolding mechanism with multiple electrodes (3 electrodes) to deliver RF energy for reducing the technical failures of RDN. The purpose of this study was to evaluate the safety and efficacy of the DENEX™ system in resistant hypertension.
METHODS
Study population
Patients (≥20 years of age) who were referred by a primary physician or a hypertension specialist to the 5 participating institutes were screened for enrollment. The inclusion criteria were 1) office systolic blood pressure (SBP) ≥150 mmHg and 2) daytime ambulatory SBP ≥140 mmHg with 3 or more anti-hypertensives including angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers, calcium channel blockers, and thiazide diuretics. The major exclusion criteria were 1) hemodynamically or anatomically significant renal arterial stenosis (>50%), 2) inappropriate anatomy for the renal denervation procedure based on computed tomography (CT) or renal angiography findings, 3) renal arteries <4 or >8 mm in diameter or <20 mm in length, and 4) a diagnosis of secondary hypertension. The other inclusion and exclusion criteria are listed in
Supplementary Table 1. Patients or their legal representatives provided written informed consent. The study was approved by each institution's ethics committee and Institutional Review Board and is registered with Clinical Trials Registry (registration No.
NCT04248530). The study diagram is presented in
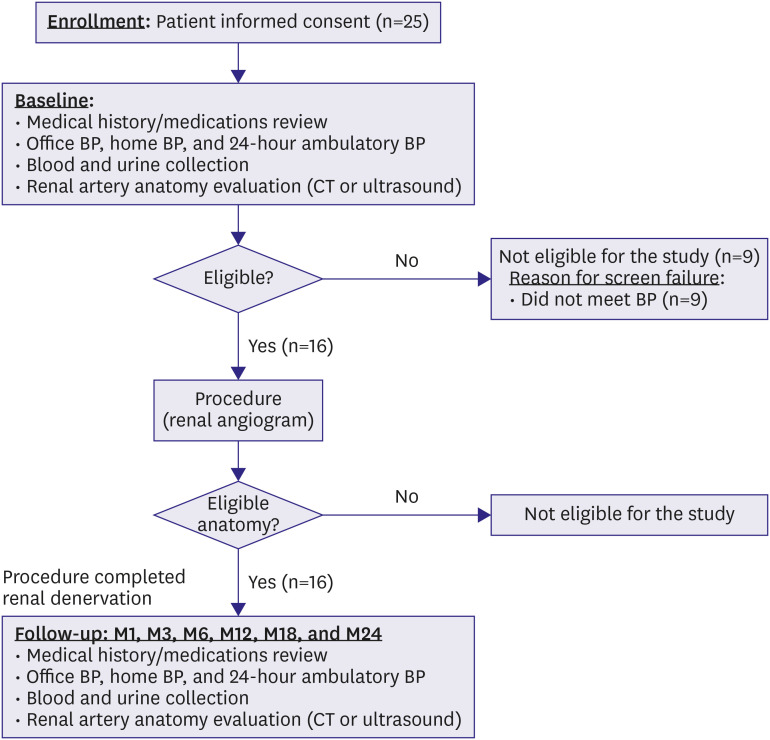
Figure 1.
Figure 1
Diagram of the DENEX pilot study.
BP = blood pressure; CT = computed tomography.

Study design
The DENEX study is the first-in-human, open-label, single-arm, multicenter pilot study to evaluate the safety and efficacy of a novel multielectrode RDN system in patients with resistant hypertension. The primary objective was safety based on all adverse events (AEs) during the procedure and follow-up period. The primary safety endpoint was the 1 month incidence of major AEs and occurrence of AEs during 3 months after the procedure. Major AEs are defined as a composite of all cause death, end-stage renal disease, embolism causing end organ damage, renal artery dissection or perforation requiring intervention, renal artery or other major vascular complications requiring intervention, hypertensive seizures requiring hospitalization, renal artery stenosis (>70%) diagnosed by angiography, renal failure (estimated glomerular filtration rate [eGFR] <15 mL/min/1.73m2, renal replacement therapy, increase in creatinine level over 2 times of baseline).
The secondary objective was efficacy based on changes at 3 months in 1) 24-hour ambulatory SBP/diastolic blood pressure (DBP); 2) office SBP/DBP; 3) percentage of patients with office SBP reduction >10, 15, and 20 mmHg; 4) percentage of patients with office SBP <140, 140–159, 160–179, and 180 mmHg; 5) heart rate (HR); 6) laboratory parameters; serum creatinine, cystatin C, brain natriuretic peptide (BNP), renin, and aldosterone, lipid profile, hemoglobin A1c, glucose, insulin, C-peptide, and urinary albumin/creatinine ratio. The definition of responder to RDN was office SBP reduction more than 10 mmHg.
DENEX™ percutaneous renal sympathetic denervation system
The DENEX™ system is a percutaneous RDN system consisting of an ablation catheter that delivers RF energy to the patients' renal arteries and a generator that produces and transmits RF energy to the catheter. RF energy is delivered to the renal arterial wall via the catheter to cause the bioimpedance to generate heat and subsequently blocks the sympathetic nerves in the outer wall of the renal arties.
DENEX™ catheter (DENEX C 002; Handok Kalos Medical, Seoul, Korea) is a single-use, foot-controlled electric handpiece and has 3 expandable electrodes separated by 120° angles and equipped with temperature sensors. It is introduced to the renal artery through a 0.014-inch guidewire and requires a >6 Fr guiding catheter (
Figure 2A). The DENEX™ generator (DENEX G 001; Handok Kalos Medical) produces and transmits RF energy to the renal arterial wall for 60 seconds at each ablation through the DENEX™ catheter. The generator supplies power (up to 10 W) to the 3 electrodes in the distal segment of the ablation catheter through 3 independent channels. The output temperature and impedance range are 10–80°C and 50–500 Ω, respectively. If the temperature exceeds 80°C, the generator shuts down the RF power (
Figure 2B).
Figure 2
DENEX™ percutaneous RDN system. (A) DENEX™ catheter. (B) DENEX™ generator.


Study procedures
Baseline and screening
After acquiring written informed consent forms from the patients, the demographic data, previous medical history including medications within 12 weeks, and physical examination results were recorded. The office BP and HR, 24-hour ambulatory BP monitoring (ABPM), and home BP were recorded during the screening period. Laboratory test data were collected and analyzed in a central laboratory.
During the screening period, office BP, home BP and ABPM were measured for eligibility for enrollment. Detailed BP measurement is described in
Supplementary Table 2. If the office BP or ABPM did not meet the inclusion criteria, the patients were not enrolled. Throughout the screening and follow-up periods, patient compliance was assessed using a medication diary filled in by the patients.
To evaluate the vascular responses of the renal arteries, duplex ultrasonogram (DUS) or abdominal CT was performed during the screening period and at 6 months after the RDN procedure.
Renal sympathetic denervation
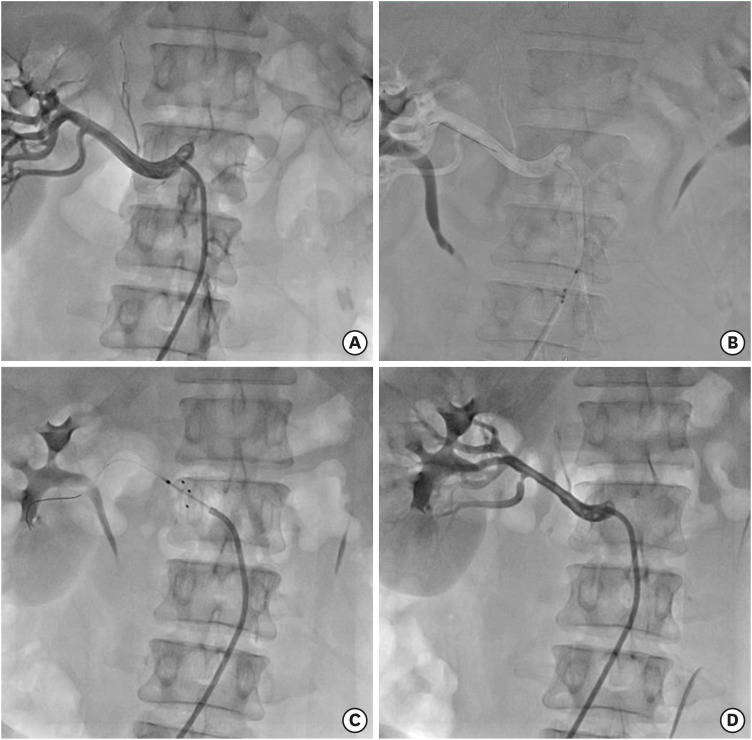
If the patients were eligible for the study during the 21-day screening period, they were taken to a catheterization laboratory for renal angiography. With the patients under conscious sedation and local anesthesia, a guiding catheter (≥7 Fr RDN or internal mammary artery catheter) was inserted via the femoral artery. If both renal arteries were appropriate for the RDN procedure, the DENEX™ catheter was advanced with a 0.014-inch guidewire up to the renal artery. After determining the site for ablation using angiography, the head of the catheter was opened and each electrode was allowed to completely contact the inner wall of the renal artery. When the catheter was stabilized at the ablation site, high-frequency energy was generated at a maximum temperature of ≤80°C, maximum output of ≤10 W, and a duration of ≤120 seconds, at the operator's discretion. If 2 or more ablation cycles were performed, the next ablation was performed at least 5 mm proximal to the previous ablation site. The number of cycles and ablation sites (i.e., main and branch renal arteries or only the main renal arteries) were subject to the operator's discretion. The same procedure was performed at the contralateral renal artery. Upon completion of renal denervation, the DENEX™ catheter and guiding catheter were sequentially removed. Finally, the femoral sheath was removed and hemostasis was achieved according to the protocol of each participating center. Procedure data, including ablation location, number, and time, were collected (
Figure 3).
Figure 3
RDN by DENEX™ percutaneous RDN system. (A) Renal angiogram for renal artery is performed. After the renal angiogram is confirmed for eligibility for RDN procedure, guiding catheter is advance to aorta and engaged in renal artery. (B) Guidewire is advanced to distal renal artery. (C) After proper position of RDN catheter is confirmed, the electrodes are opened and renal denervation is performed for 60 seconds. (D) After RDN procedure is completed in 1 of the renal arteries, renal angiogram is performed again to check for any post-ablation complication.
RDN = renal sympathetic denervation.

After the procedure, the patients were moved to the general ward or cardiac intensive care unit, and vital signs were monitored. The patients were discharged on the following day if no periprocedural complications were noted.
Follow-up
The patients were scheduled to be followed up for 24 months (1, 3, 6, 12, 18, and 24 months) after the index RDN procedure. During the follow-up period, the patients were advised to continue their current antihypertensives. Throughout the follow-up period, data on AEs were collected. Office BP was recorded at 1, 3, 6, 12, 18, and 24 months, whereas ABPM and home BP were recorded at 3, 6, 12, 18, and 24 months. A follow-up renal imaging study, either DUS or abdominal CT, was performed at 6 months after the RDN procedure. Laboratory tests were performed at 3, 12, and 24 months. The medication diaries were examined at 3, 6, 12, and 18 months.
Bailout treatment
The patients were requested to continue their current antihypertensives for at least 6 months after RDN. The medications could be changed at the investigator's discretion after 6 months. However, under the following conditions, the medications could be changed.
1) Office SBP ≥180 mmHg and ambulatory daytime SBP ≥170 mmHg (within 21 days of office BP measurement) after RDN;
2) Office SBP <100 mmHg and ambulatory daytime SBP <95 mmHg (within 21 days of office BP measurement) after RDN;
3) Reduction of office SBP >20 mmHg compared to baseline office SBP with symptoms of hypotension and ambulatory daytime SBP >15 mmHg compared to baseline; and
4) Adverse effects from current antihypertensives necessitating medication change.
Statistical analysis
As this study is the first-in-human study to evaluate the safety of RDN with the DENEX™ system, sample size calculation was not performed. Analysis of demographic and other baseline characteristics was conducted on the full analysis set. The primary objective was safety based on AEs, which was analyzed using raw data without imputation for missing values. AEs were coded according to Medical Dictionary for Regulatory Activities (MedDRA) and classified as system organ class and preferred term. All AEs were classified according to severity, and AEs associated with the renal denervation system and major AEs are separately presented. The major AEs were 1) all-cause death; 2) severe kidney injury 3) any embolism causing organ damage (i.e., renal/intestinal infarction, ulcer, or gangrene of the lower extremities); 4) renal arterial perforation or dissection necessitating interventional treatment; 5) vascular complications (inguinal hematoma, arteriovenous fistula, or pseudo-aneurysm) necessitating surgery, interventional treatment, thrombin injection, or transfusion; and 6) hypertensive crisis unrelated to noncompliance to antihypertensives. AEs were reported by each investigator and adjudicated by an independent data monitoring committee. The secondary objective is presented as changes from baseline and analyzed using a paired t-test. Missing values were adjusted using the last observation carried forward approach and analyzed using raw data without imputation for missing values. For continuous data, the number of patients, mean, standard deviation, median, minimum, and maximum values are separately presented. For categorical data, frequencies and percentages (%) are presented as descriptive statistics. For continuous data, the statistical significance of the differences before and after the treatment was tested using an independent t-test or the Wilcoxon rank sum test. For categorical data, the statistical significance of the differences before and after the treatment was tested using the χ2 test or Fisher's exact test. A 2-tailed p value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
A total of 25 patients were screened for eligibility, and 9 patients were excluded because they did not meet the BP criteria either by office BP or ABPM. The remaining 16 patients underwent renal angiography and proceeded to RDN. Five patients underwent RDN in both the main and branch renal arteries. A total of 13 patients completed the 24-month follow-up (3 patients dropped out after being followed up for 1 month).
Demographic and procedural data
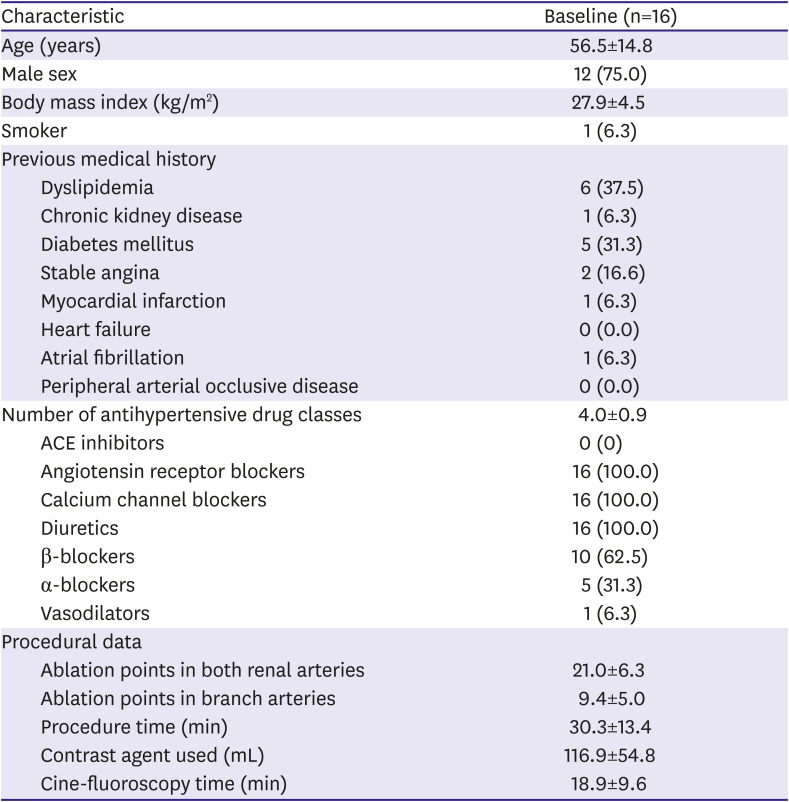
The mean age of the patients was 55.6±14.9 years, and 75% (n=12) were men. The mean body mass index was 28.0±4.5 kg/m2, and 1 patient was a smoker. One patient had chronic kidney disease, 5 patients had diabetes, and 6 patients had dyslipidemia. Two patients had a history of stable angina, 1 patient had a history of myocardial infarction, and 1 patient had a history of atrial fibrillation. The mean number of antihypertensives was 4.0±0.9. All patients were using angiotensin receptor blockers, calcium channel blockers, and diuretics. Additionally, 10 patients were using beta-blockers. The mean age at diagnosis was 40.1±9.6 years, and the mean duration of hypertension was 15.9±12.6 years.
The mean number of ablation points in both renal arteries was 21.0±6.3 and that in branch arteries was 9.4±5.0. The mean procedure time was 30.3±13.4 minutes, and the contrast amount used was 116.9±54.9 mL. Eleven patients underwent RDN only in the main renal arteries, and 5 patients underwent RDN both in the main and branch renal arteries (
Table 1).
Table 1
Baseline characteristics
|
Characteristic |
Baseline (n=16) |
|
Age (years) |
56.5±14.8 |
|
Male sex |
12 (75.0) |
|
Body mass index (kg/m2) |
27.9±4.5 |
|
Smoker |
1 (6.3) |
|
Previous medical history |
|
|
Dyslipidemia |
6 (37.5) |
|
Chronic kidney disease |
1 (6.3) |
|
Diabetes mellitus |
5 (31.3) |
|
Stable angina |
2 (16.6) |
|
Myocardial infarction |
1 (6.3) |
|
Heart failure |
0 (0.0) |
|
Atrial fibrillation |
1 (6.3) |
|
Peripheral arterial occlusive disease |
0 (0.0) |
|
Number of antihypertensive drug classes |
4.0±0.9 |
|
ACE inhibitors |
0 (0) |
|
Angiotensin receptor blockers |
16 (100.0) |
|
Calcium channel blockers |
16 (100.0) |
|
Diuretics |
16 (100.0) |
|
β-blockers |
10 (62.5) |
|
α-blockers |
5 (31.3) |
|
Vasodilators |
1 (6.3) |
|
Procedural data |
|
|
Ablation points in both renal arteries |
21.0±6.3 |
|
Ablation points in branch arteries |
9.4±5.0 |
|
Procedure time (min) |
30.3±13.4 |
|
Contrast agent used (mL) |
116.9±54.8 |
|
Cine-fluoroscopy time (min) |
18.9±9.6 |

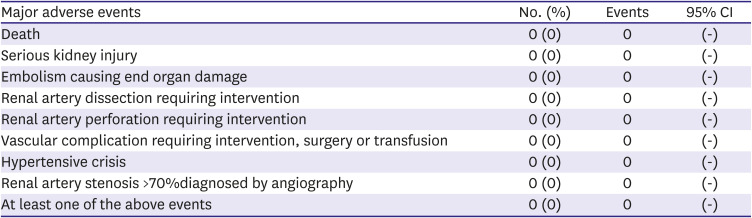
Primary objective: safety
During the study period 6 months, no major AEs occurred, including death, serious kidney injury, or renal or access artery complications requiring intervention or surgery. In the follow-up DUS or abdominal CT, no renal arterial stenosis >70% was observed in the ablated arteries. A total of 22 non-major AEs were reported in 9 patients. In laboratory investigations, mildly elevated blood creatinine, presence of albuminuria, decrease in renin, and increase in aldosterone were reported. With respect to vascular disorders, hypertension, hypotension, or peripheral coldness were reported in 2 patients. One patient reported back pain, and 1 patient reported urinary retention. Fifteen non-major adverse device effects were reported. In laboratory investigations, increased blood aldosterone and decreased renin were reported in 2 patients (
Table 2).
Table 2
Safety profile in association with the DENEX™ RDN system (n=16)
|
Major adverse events |
No. (%) |
Events |
95% CI |
|
Death |
0 (0) |
0 |
(-) |
|
Serious kidney injury |
0 (0) |
0 |
(-) |
|
Embolism causing end organ damage |
0 (0) |
0 |
(-) |
|
Renal artery dissection requiring intervention |
0 (0) |
0 |
(-) |
|
Renal artery perforation requiring intervention |
0 (0) |
0 |
(-) |
|
Vascular complication requiring intervention, surgery or transfusion |
0 (0) |
0 |
(-) |
|
Hypertensive crisis |
0 (0) |
0 |
(-) |
|
Renal artery stenosis >70%diagnosed by angiography |
0 (0) |
0 |
(-) |
|
At least one of the above events |
0 (0) |
0 |
(-) |

Secondary objective: efficacy
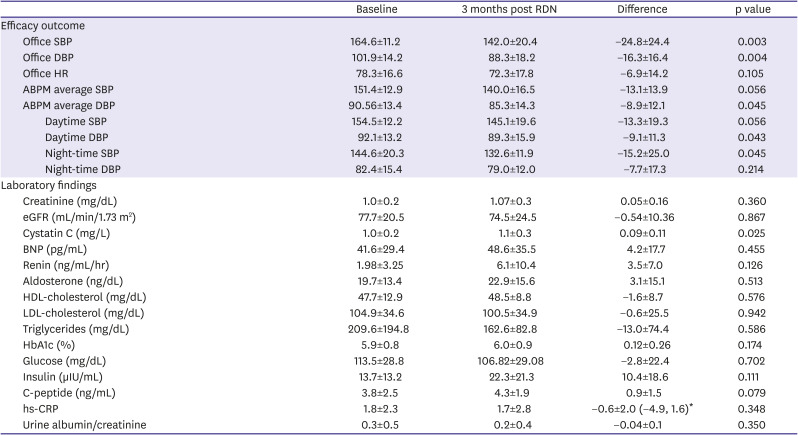
The average baseline ambulatory SBP and DBP were 151.4±12.8 and 90.5±13.4 mmHg, respectively, and the baseline office SBP and DBP were 164.6±11.2 and 101.9±14.2 mmHg, respectively. At 3 months, the ambulatory SBP and DBP decreased by 13.1 mmHg (p
=0.056) and 8.9 mmHg (p=0.045), respectively. The office SBP and DBP decreased by 24.8 mmHg (p=0.003) and 16.3 mmHg (p=0.004), respectively (
Table 3,
Figure 4). The percentage of office SBP decrease >10 mmHg was 92.3% (n=12), that of office SBP decrease >15 mmHg was 76.9% (n=10), and that of office SBP decrease >20 mmHg was 46.2% (n=6) at 3 months. The percentage of office SBP <140 mmHg was 38.5% (95% CI, 13.86–68.42; n=5), that of SBP 140–159 mmHg was 38.46% (95% CI, 13.86–68.42; n=5), and that of SBP 160–179 mmHg was 23.1% (95% CI, 5.04–53.81; n=3) at 3 months (
Figure 5). The mean baseline HR was 78.3±16.6 beats/min and decreased to 72.4±17.8 beats/min without significance (p
=0.105).
Figure 4
Ambulatory and office BP reduction from baseline to 3 months.
ABPM = ambulatory blood pressure monitoring; BP = blood pressure; SBP = systolic blood pressure.
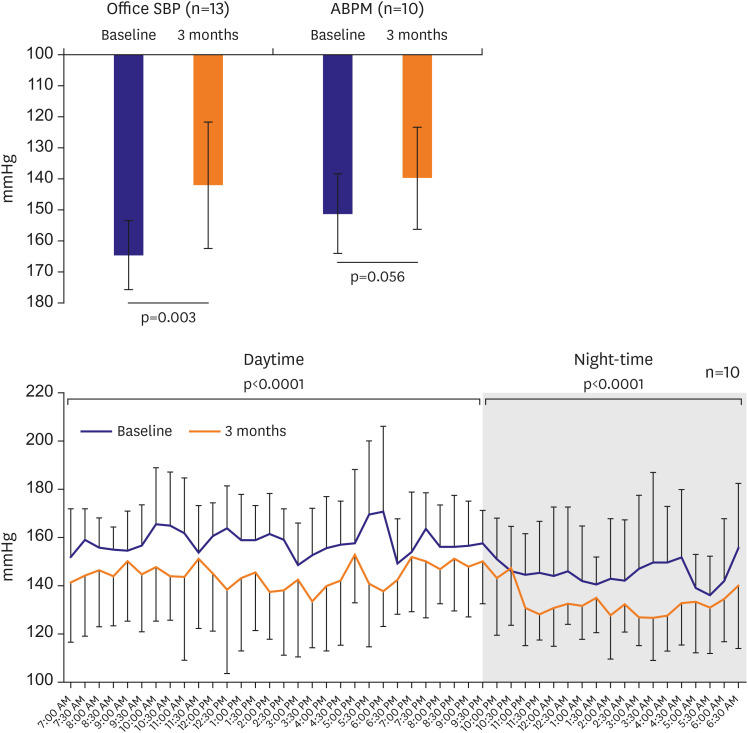

Figure 5
BP reduction at 3 months and composition of BP.
BP = blood pressure.
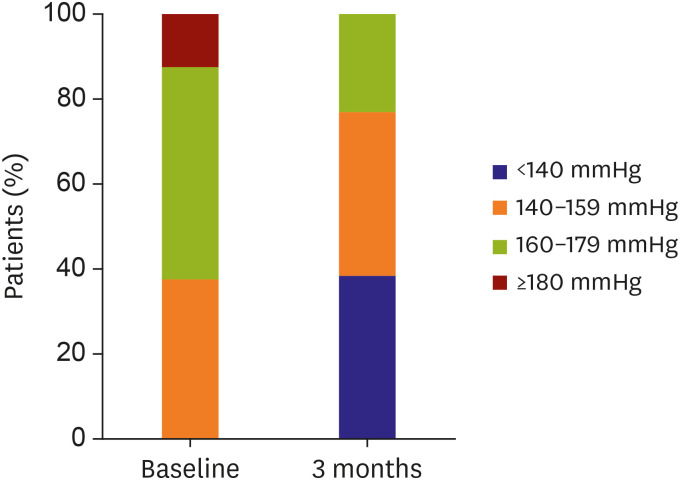

Table 3
Change of efficacy outcome and laboratory findings from baseline to 3 months post RDN
|
Baseline |
3 months post RDN |
Difference |
p value |
|
Efficacy outcome |
|
|
|
|
|
Office SBP |
164.6±11.2 |
142.0±20.4 |
−24.8±24.4 |
0.003 |
|
Office DBP |
101.9±14.2 |
88.3±18.2 |
−16.3±16.4 |
0.004 |
|
Office HR |
78.3±16.6 |
72.3±17.8 |
−6.9±14.2 |
0.105 |
|
ABPM average SBP |
151.4±12.9 |
140.0±16.5 |
−13.1±13.9 |
0.056 |
|
ABPM average DBP |
90.56±13.4 |
85.3±14.3 |
−8.9±12.1 |
0.045 |
|
|
Daytime SBP |
154.5±12.2 |
145.1±19.6 |
−13.3±19.3 |
0.056 |
|
|
Daytime DBP |
92.1±13.2 |
89.3±15.9 |
−9.1±11.3 |
0.043 |
|
|
Night-time SBP |
144.6±20.3 |
132.6±11.9 |
−15.2±25.0 |
0.045 |
|
|
Night-time DBP |
82.4±15.4 |
79.0±12.0 |
−7.7±17.3 |
0.214 |
|
Laboratory findings |
|
|
|
|
|
Creatinine (mg/dL) |
1.0±0.2 |
1.07±0.3 |
0.05±0.16 |
0.360 |
|
eGFR (mL/min/1.73 m2) |
77.7±20.5 |
74.5±24.5 |
−0.54±10.36 |
0.867 |
|
Cystatin C (mg/L) |
1.0±0.2 |
1.1±0.3 |
0.09±0.11 |
0.025 |
|
BNP (pg/mL) |
41.6±29.4 |
48.6±35.5 |
4.2±17.7 |
0.455 |
|
Renin (ng/mL/hr) |
1.98±3.25 |
6.1±10.4 |
3.5±7.0 |
0.126 |
|
Aldosterone (ng/dL) |
19.7±13.4 |
22.9±15.6 |
3.1±15.1 |
0.513 |
|
HDL-cholesterol (mg/dL) |
47.7±12.9 |
48.5±8.8 |
−1.6±8.7 |
0.576 |
|
LDL-cholesterol (mg/dL) |
104.9±34.6 |
100.5±34.9 |
−0.6±25.5 |
0.942 |
|
Triglycerides (mg/dL) |
209.6±194.8 |
162.6±82.8 |
−13.0±74.4 |
0.586 |
|
HbA1c (%) |
5.9±0.8 |
6.0±0.9 |
0.12±0.26 |
0.174 |
|
Glucose (mg/dL) |
113.5±28.8 |
106.82±29.08 |
−2.8±22.4 |
0.702 |
|
Insulin (µIU/mL) |
13.7±13.2 |
22.3±21.3 |
10.4±18.6 |
0.111 |
|
C-peptide (ng/mL) |
3.8±2.5 |
4.3±1.9 |
0.9±1.5 |
0.079 |
|
hs-CRP |
1.8±2.3 |
1.7±2.8 |
−0.6±2.0 (−4.9, 1.6)*
|
0.348 |
|
Urine albumin/creatinine |
0.3±0.5 |
0.2±0.4 |
−0.04±0.1 |
0.350 |

The baseline creatinine level and estimated glomerular filtration rate were 0.99±0.27 mg/dL and 77.04±21.6 mL/min/1.73 m
2, respectively, and the follow-up values at 3 months were 1.07±0.34 mg/dL and 74.5±24.5 mL/min/1.73 m
2, respectively, without a significant difference (p=0.360, p=0.867). The baseline cystatin C level was 1.05±0.29 mg/dL and increased by 0.09±0.11 mg/dL (p=0.025). The baseline BNP, renin, and aldosterone levels were 41.6±29.4 pg/mL, 1.98 ng/mL/h, and 19.66 ng/dL, respectively, with no significant difference at 3 months (p=0.455, p=0.126, and p=0.513, respectively) (
Table 3).
DISCUSSION
The present study demonstrated that the DENEX™ RDN system is safe and effective in the treatment of resistant hypertension. No major AEs such as death, significant kidney injury, or renal or access artery complications occurred. Minor AEs were reported in 9 patients; however, most of the events were deemed not or unlikely to be associated with the RDN procedure.
The first-generation RDN device (Symplicity
® flex catheter) demonstrated the efficacy and safety of percutaneous RDN as a treatment modality for resistant hypertension in previous trials.
3)4) One case of renal dissection and one case of pseudoaneurysm of the access artery were reported in Symplicity-1.
3) Meanwhile, one case of femoral artery pseudoaneurysm, one case of urinary tract infection, one case of extended admission due to paresthesia, and one case of back pain were reported in Symplicity-2.
4) Another RDN trial with a multielectrode ablation catheter reported no serious vascular AEs, but reported hypotension, progression of preexisting renal artery stenosis, and progression of hypertensive renal disease with an increase in serum creatinine.
13) The AEs reported in the present study are consistent with those in previous studies.
A previous study with a single-electrode catheter failed to show the efficacy of the procedure in a sham-controlled clinical trial.
7) Analysis of Symplicity-3 suggested that insufficient number of ablations and failure to ablate the full circumference of the renal arteries may have contributed to the negative results.
8)9)
The DENEX™ RDN system was developed to ensure full circumference ablation and sufficient ablation number to decrease renal sympathetic activities. To evaluate the in vivo safety and efficacy, preclinical evaluation was performed with minipig models from the early stage of development at the Cardiovascular Product Evaluation Center at Yonsei University. In addition, a good laboratory practice (GLP) safety study of RDN was performed using minipigs and neither death nor abnormality of the renal arteries or renal function was observed during the study period.
In this study, the DENEX™ RDN system was able to perform full-circumference ablation through the 3 electrodes, and ensured firm wall contact and increased ablation numbers. The mean ablation number in this study was 21.0±6.3, which is greater than 9.2±2.0 in Symplicity-3. This increased number might have contributed to the greater reductions in both ambulatory SBP (−13.10±18.89 vs. −6.75±15.11 mmHg) and office SBP (−24.19±20.41 vs. −14.13±23.93 mmHg) than those in Symplicity-3.
7)
Another study (EnligHTN study) that adopted a basket-type device for percutaneous RDN showed comparable results to those of our study. BP reduction was similar to that in our study, with ambulatory and office SBP reduction at 3 months of −10 and −27 mmHg, respectively.
13)
Of note, procedure time of DENEX™ RDN system was shorter compared to EnlighHTN study. Unlike the DENEX™ RDN system, the EnligHTN device did not employ a 0.014-in guidewire and did not perform ablation in distal renal arteries. As the renal sympathetic nerve is located closer to the renal arterial wall in distal renal arteries,
14) the ability to advance and ablate in distal renal arteries may influence the efficacy of an RDN system. In our study, 9 ablation points in 5 patients were targeted in distal renal arteries. The effect of the difference may need to be discussed in further studies.
Recently published studies emphasized that sufficient ablation number and full-circumference ablation were essential for achieving significant BP reduction in resistant hypertension. In the SPYRAL HTN-OFF MED proof-of-concept study, which adopted the Symplicity Spyral multielectrode catheter, ensuring circumferential energy delivery, an average of 43.8±13.1 ablations were performed and achieved significant ambulatory SBP reduction compared with the sham group.
15) In the SPYRAL HTN-ON MED proof-of-concept trial, similar ablation numbers of 45.9±13.7 per patient achieved significant reduction of ambulatory BP. Additionally, a significant number of ablations were performed in branch arteries in these studies, and as the renal sympathetic nerves are located closer to the distal renal arteries than the main renal arteries, this ablation strategy might have contributed to the significantly larger BP reduction than in the sham group. In our study, DENEX™ RDN was able to advance to and ablate the distal renal arteries using a 0.014-in guidewire. Distal renal artery ablation was performed at the operator's discretion, and 5 of 16 patients had both the main and branch arteries ablated. The ability to access the distal renal arteries may enhance the efficacy of the DENEX™ RDN system.
In the most recently published pivotal study, the SPYRAL RDN system showed statistically significant BP reduction compared with a sham group off medication.
16) The total ablation number and ablation in branch arteries were similar to those in the previous SPYRAL HTN OFF and ON studies, and repeatedly showed the importance sufficient number of ablations and the location of ablation.
After success of recent trials, application of RDN beyond hypertension, such as arrhythmia,
17) heart failure,
18) or left ventricular remodeling are being investigated. With use of novel RDN systems, such as DENEX™ RDN, we anticipate RDN can expand its clinical utilization.
The limitation of our study was the relatively small number of enrolled patients, which might have underestimated the AEs. However, our study showed similar or lower AE rates relative to other studies.
3)4)13) Additionally, most patients completed the 24-month follow-up and the AE rates did not increase over time. The small sample size may have also influenced the assessment of efficacy. Although the amount of BP reduction was similar to that of other studies,
3)4)13) the ambulatory SBP reduction was marginally significant. The assessment of efficacy should be evaluated in larger-scale randomized controlled trials.
Additionally, the assessment of compliance is limited due to poor submission of medication diary from the patients. Future study using DENEX™ RDN system needs to address this issue.
In conclusion, the present study showed the safety and efficacy of the DENEX™ RDN system for patients with resistant hypertension. Further assessment of statistically significant and clinically meaningful reduction in hypertension should be evaluated in future large-scale studies.









 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download