This article has been
cited by other articles in ScienceCentral.
Abstract
Closure of the left atrial appendage using percutaneous transcatheter occlusion devices is used for stroke prevention as an alternative for patients with a high risk or contraindications for long-term oral anticoagulation use. Herein, we will discuss the practical aspects of five among the available devices used for interventional left atrial appendage occlusion: Watchman, Amulet, WaveCrest, LAmbre, and Lariat.
Go to :

Keywords: Left atrial appendage occlusion, Stroke
INTRODUCTION
The most worrisome and ominous consequence of atrial fibrillation (AF) remains thromboembolism, most importantly stroke.
1) The left atrial appendage (LAA) remains a focus of thrombus formation in patients with AF and surgical amputation of the LAA during open chest procedures for the explicit purpose of reducing stroke risk in AF patients has been first described in the 1950s.
2)3)4) Nowadays, surgical closure of the LAA is included in the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for management of patients with valvular heart disease undergoing heart surgery.
5) Moreover, ACC/AHA/Heart Rhythm Society (HRS) guidelines for cardiac surgery patients with a history of AF include surgical LAA closure whenever possible.
6) Historically, stroke prevention has been focused on systemic pharmacologic anti-thrombotic strategies: oral anticoagulation (OAC) with warfarin or the novel oral anticoagulants are the agents of choice for reducing stroke risk in patients with higher risk factors based on their risk scores (CHADS
2, CHA
2DS
2-VASc). However, OACs might be ineffective due to the concern of major bleeding and the consequences of pharmacological non-compliance. Therefore, non-pharmacologic options are attractive alternatives for patients with a high stroke risk and contraindications for long-term OAC use. Among these, LAA occlusion (LAAO) using percutaneous implanted devices is recommended by the current AHA/ACC/HRS and European Heart Rhythm Association (EHRA)/European Association of Percutaneous Cardiovascular Interventions (EAPCI) guidelines.
7)8) These recommendations derive from numerous prospective observational registries that have shown the feasibility and relative safety of this approach utilizing different devices, as well as two randomized controlled trials that have shown non-inferiority of LAAO with Watchman when compared to chronic warfarin in terms of stroke, systemic embolism, or death (cardiovascular/unexplained).
9)10)
Herein, we will discuss the practical aspects of five among the available devices that provide percutaneous transcatheter closure of the LAA (
Table 1;
Figure 1): Watchman, Amulet, WaveCrest, LAmbre, and Lariat. Their main clinical studies are summarized in
Table 2, with a focus on peri-procedural complications and feasibility of complete LAAO.
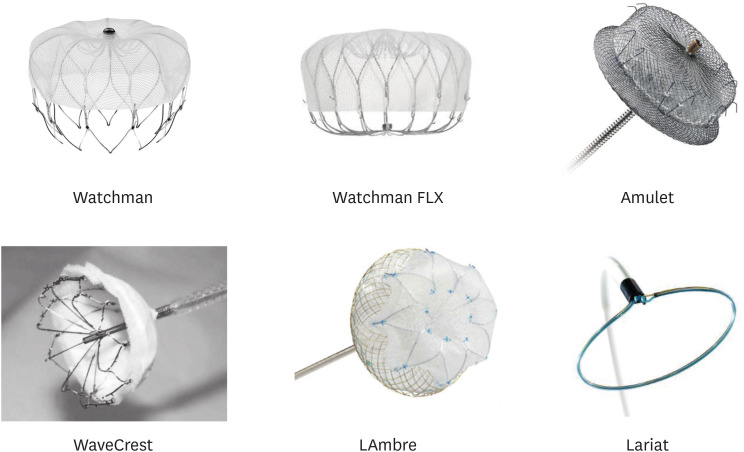 | Figure 1 Principal devices for left atrial appendage closure.
|
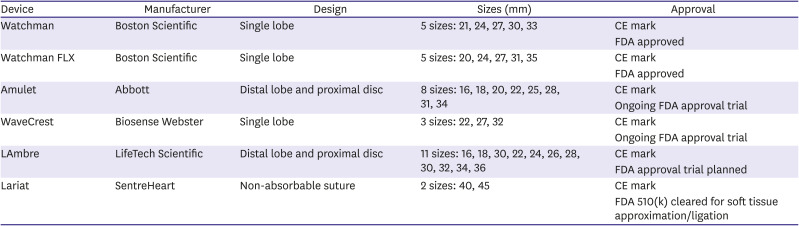
Table 1
Left atrial appendage occlusion devices, main characteristics
|
Device |
Manufacturer |
Design |
Sizes (mm) |
Approval |
|
Watchman |
Boston Scientific |
Single lobe |
5 sizes: 21, 24, 27, 30, 33 |
CE mark |
|
FDA approved |
|
Watchman FLX |
Boston Scientific |
Single lobe |
5 sizes: 20, 24, 27, 31, 35 |
CE mark |
|
FDA approved |
|
Amulet |
Abbott |
Distal lobe and proximal disc |
8 sizes: 16, 18, 20, 22, 25, 28, 31, 34 |
CE mark |
|
Ongoing FDA approval trial |
|
WaveCrest |
Biosense Webster |
Single lobe |
3 sizes: 22, 27, 32 |
CE mark |
|
Ongoing FDA approval trial |
|
LAmbre |
LifeTech Scientific |
Distal lobe and proximal disc |
11 sizes: 16, 18, 30, 22, 24, 26, 28, 30, 32, 34, 36 |
CE mark |
|
FDA approval trial planned |
|
Lariat |
SentreHeart |
Non-absorbable suture |
2 sizes: 40, 45 |
CE mark |
|
FDA 510(k) cleared for soft tissue approximation/ligation |

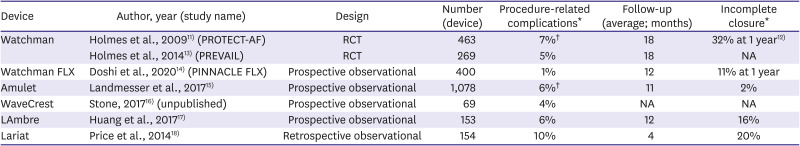
Table 2
Left atrial appendage occlusion devices, main studies (feasibility, safety)
|
Device |
Author, year (study name) |
Design |
Number (device) |
Procedure-related complications*
|
Follow-up (average; months) |
Incomplete closure*
|
|
Watchman |
Holmes et al., 200911) (PROTECT-AF) |
RCT |
463 |
7%†
|
18 |
32% at 1 year12)
|
|
Holmes et al., 201413) (PREVAIL) |
RCT |
269 |
5% |
18 |
NA |
|
Watchman FLX |
Doshi et al., 202014) (PINNACLE FLX) |
Prospective observational |
400 |
1% |
12 |
11% at 1 year |
|
Amulet |
Landmesser et al., 201715)
|
Prospective observational |
1,078 |
6%†
|
11 |
2% |
|
WaveCrest |
Stone, 201716) (unpublished) |
Prospective observational |
69 |
4% |
NA |
NA |
|
LAmbre |
Huang et al., 201717)
|
Prospective observational |
153 |
6% |
12 |
16% |
|
Lariat |
Price et al., 201418)
|
Retrospective observational |
154 |
10% |
4 |
20% |

Go to :

PROCEDURE (GENERAL CONSIDERATIONS)
Pre-procedural imaging is essential to exclude LAA thrombus and assess LAA anatomy for suitability for percutaneous closure and to determine appropriate sizing of the chosen device (see relative sections). With transesophageal echocardiography (TEE), the LAA is visualized in multiple planes (most commonly, using 0°, 45°, 90°, and 135° angles). In these views, it is possible to determine the maximal LAA dimensions relevant to the intravascular LAAO device used (
Figure 2). In alternative, computed tomography can be employed: its superior spatial resolution allows for 3D reconstructions, and LAA thrombi can be safely ruled out with delayed acquisition imaging.
20)
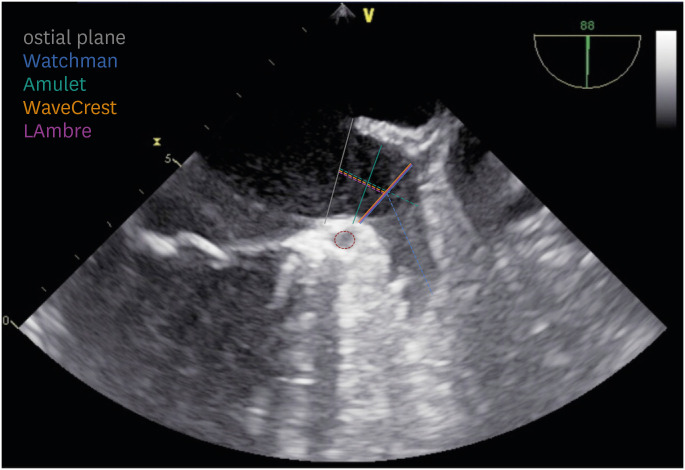 | Figure 2
Transesophageal echocardiography for sizing of intravascular LAAO devices. Examples of different LAA measurements on a 90° TEE view. Solid and dashed lines represent LAA landing zone and depth, respectively; grey solid line represents the anatomical ostium while the red dashed circle is the left circumflex coronary artery. Reproduced with permission from Gianni et al.19)
LAA = left atrial appendage; LAAO = left atrial appendage occlusion; TEE = transesophageal echocardiography.

|
To minimize embolic risk, the procedure should be performed while on uninterrupted OAC (therapeutic warfarin or factor Xa inhibitors)
21) and heparin should be administered before transseptal access, (goal activated clotting time [ACT] more than 250 seconds at the time of left atrial [LA] instrumentation). The procedure itself is usually performed under general anesthesia and guided by fluoroscopy and TEE. Intracardiac echocardiography (ICE) is a safe and effective alternative to TEE: it allows for more precise trans-septal access and within the LA provides suitable near-field views of the LAA.
22) For most LAAO procedures, the transseptal puncture should be low, usually anterior (the LAA should be seen on ICE), to facilitate engagement of the LAA (
Figure 3). The latter should be done using a pigtail catheter and contrast, to reduce the risk of LAA laceration. Once the LAA is engaged, angiography is performed to confirm the LAA size, usually using a right anterior oblique (RAO) caudal projection, roughly corresponding to a 135° TEE view. Additional views might be needed for proper sizing and safe sheath positioning (i.e., antero-posterior [AP] cranial, RAO cranial, and RAO corresponding to a 0°, 45°, and 90° TEE view, respectively). For intravascular LAAO devices, deployment is performed in breath-hold, which minimizes the risk of LAA laceration during unsheathing/advancement.
 | Figure 3
Importance of a low mid-to-anterior transseptal puncture. For LAAO the transseptal puncture should be low, in the mid to anterior septum to facilitate engagement of the LAA. On ICE (rightmost), the LAA and MV should be in view. Reproduced with permission from Gianni et al.19)
IAS = interatrial septum; ICE = intracardiac echocardiography; LAA = left atrial appendage; LAAO = left atrial appendage occlusion; MV = mitral valve.

|
After the procedure, the patient must be placed on a combination of anti-thrombotic medications (antiplatelet and/or anticoagulation), with the specific regimen based on the LAAO device used. Surveillance TEE imaging is required to confirm adequate LAAO at follow-up: incomplete occlusion (i.e., presence of peri-device leaks) or device thrombus are usually considered an indication for continued treatment with OAC. Of note, peri-device leaks can be closed in a “repeat” transcatheter LAAO procedure using vascular plugs, coils, or septal occluder devices.
23)
In the following sections each device will be discussed as to their specific technical and procedural aspects.
Go to :

WATCHMAN
The Watchman FLX (Boston Scientific, Marlborough, MA, USA) LAAO device is the new generation Watchman device. The Watchman FLX is a single lobe device that consists of a self-expanding nitinol 18-struts metal frame covered by a porous (160 μm) polyethylene terephthalate (PET) knit fabric (
Figure 1). The PET membrane covers the proximal surface; the portion which remains in contact with the blood of the LA cavity and promotes healing and endothelialization. Compared to the previous version, the proximal face is flat, with a recessed threaded insert to reduce the risk of device-related thrombosis. Around the frame nitinol perimeter, 12 fixation hooks are present across two lines to anchor the device to the LAA and ensure stability, which is also aided by the moderate radial force exerted by the self-expanding frame. Finally, the distal end is closed with a relatively short device length (10–20% less than the previous generation), to limit the risk of trauma within the LAA.
The device is packaged in a pre-loaded delivery sheath and is placed in the LAA by means of a 14-F access sheath. The access sheaths are provided in 3 shapes: single, double, and anterior curve. Selection of the access sheath is usually physician preference, although the double and anterior curves are better suited for LAAs with a more superior direction (towards the aortic root). The device is manufactured in 5 sizes (20, 24, 27, 31, and 35 mm) to accommodate most LAA ostial dimensions. It should be mentioned that the larger devices are also longer: as a result, a LAA with a wider ostial diameter would also need a longer body length to provide adequate sheath position prior to device deployment.
The previous generation (
Figure 1), which is still commercially available, comes in different sizes (21, 24, 27, 30, and 33 mm), which can cover a smaller range of LAA ostia. Another difference is the open distal end, potentially more traumatic during device deployment. With this design, bigger devices are also proportionally longer: as a result, a LAA with a wider ostial diameter would also need a longer body length to provide adequate sheath positioning prior to device deployment. Additionally, the nitinol frame is composed of 10 struts, with 10 anchors in a single line, which provide less radial strength with a potentially reduced device stability. Finally, the proximal facing tethered insert is not flat, and more metal remains in contact with the systemic circulation.
For Watchman FLX, it is important to obtain two LAA dimensions (
Figure 2):
• LAA landing zone width, measured from the circumflex artery to the transition between the smooth LA and the trabeculated LAA (usually 1 to 2 cm distal to the limbus of the LAA-left superior pulmonary vein ridge): between 15 and 32 mm (17 and 31 mm with Watchman)
• LAA depth, measured from the device landing zone line to apex of the LAA: at least ½ of the device size (equal or greater than the landing zone width with Watchman)
Oversizing should be by 10 to 20% to ensure stable device positioning. Of note, excessive oversizing may result in compression of the circumflex artery and should be avoided. Oversizing of Watchman FLX might also result in device embolization: with the closed distal end, it leads to reduced anchor/frame-tissue contact, thus affecting the device stability.
For Watchman FLX deployment (
Figure 4), the access sheath is advanced over the pigtail catheter aligning the appropriate radiopaque marker band (according to the device size) at the ostium of the LAA. The device (within its delivery sheath) is advanced until the fluoroscopic marker of the delivery system is aligned to the distal marker of the access sheath. At this point, the access and delivery sheath are slowly withdrawn while maintaining the device in place until it assumes a “ball” configuration. Deployment can be completed by further unsheathing the device or carefully advancing it until it's fully open. Before device release, ultrasound/fluoroscopy are used to confirm:
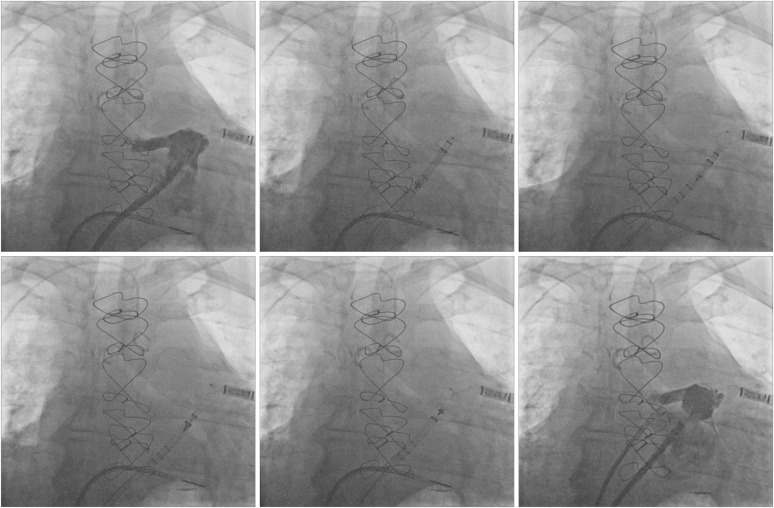 | Figure 4 Watchman FLX deployment. Step-by-step procedure as seen on fluoroscopy. See text for description.
|
• Proper positioning: the maximum device diameter should be at or just distal to the LAA ostium, not protruding too far into the LA
• Stability: assessed with a tug test, i.e., gentle traction until tactile feedback (sensation of heartbeat) and mild device deformity are demonstrated
• Correct size: the device widest diameter should be compressed 10 to 20% of its original size
• Proper occlusion: all LAA lobes should be distal to the proximal face of the device, with no peri-device flow seen on contrast injection/color Doppler
If deployment is too distal or too proximal, the device may be partially recaptured by maintaining the device in position while advancing the sheath over the device until it is shaped into the atraumatic ball. If sheath repositioning is necessary, full recapture is also possible. This is different from the previous generation (
Figure 5), where only distal deployment can be corrected with partial recapture and retreat of the sheath/device apparatus. During partial recapture, the sheath is carefully advanced over the device up to the level of the fixation hooks. If full recapture (beyond the fixation hooks) is necessary, the older generation Watchman needs to be discarded, and the procedure starts over with a new device. Once LAAO is satisfactory, the device can be released by unscrewing the connector wire.
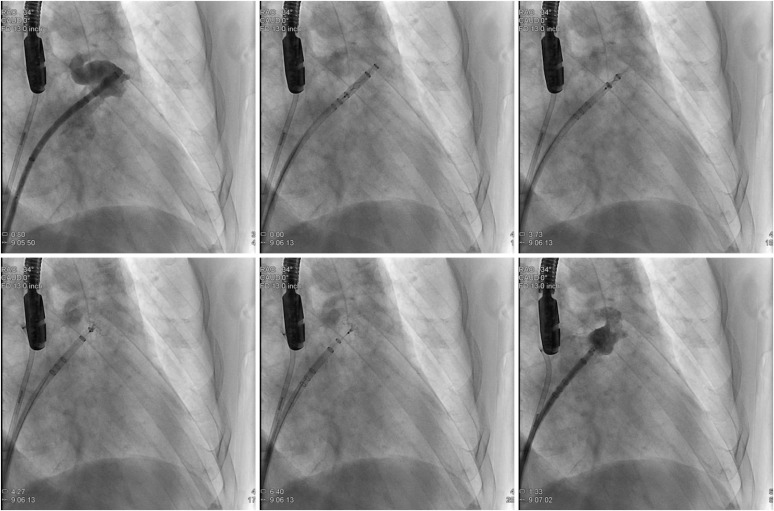 | Figure 5 Watchman deployment. Step-by-step procedure as seen on fluoroscopy. See text for description. Reproduced with permission from Gianni et al.19)

|
Go to :

AMULET
The Amulet (Abbott, Chicago, IL, USA) LAAO device consists of a self-expanding nitinol metal mesh conformed into a distal lobe and a proximal disc connected by a short, central waist (
Figure 1). The distal lobe sits within the body of the LAA while the proximal disc covers the LAA ostium from within the LA, thus providing a double sealing system. To reduce device-related thrombosis, both disc and lobe are covered by a sew-in polyester patch membrane, and the proximal end screw is recessed and tethered. The distal lobe has 6 to 10 pairs of stabilizing wires/hooks across its diameter designed anchor to device the LAA and ensure its stability, which is also aided by its mild radial force and proximal disc traction. The device is packaged in a pre-loaded delivery sheath and is placed in the LAA by means of a 12-F to 14-F double-curved access sheath. The device is manufactured in 8 sizes (according to the lobe diameter: 16, 18, 20, 22, 25, 28, 31, and 34 mm). The disc diameter is 6 to 7 mm greater than the distal lobe, with a lobe and waist length of 7.5 to 10 mm and 5.5 to 8 mm, respectively. Thus, the length of the device is shorter than its diameter, accommodating shallower LAAs.
Amulet device sizing is based on (
Figure 2):
• LAA landing zone width, measured around 1.5 cm distal to the ostial plane: between 11 and 31 mm
• LAA depth, measured from the ostial plane to the back of the LAA, following the axis of the neck: greater than 12 to 15 mm (according to the device size)
An oversizing of 2 to 4 mm is usually recommended for improved device stability. As with Watchman, excessive oversizing should be avoided, as it may result in compression of the circumflex artery and device embolization.
For Amulet deployment (
Figure 6), the access sheath is advanced over the pigtail catheter until its tip lies at the level of landing zone. This is best done using a RAO caudal projection, but different views might be used according to the specific anatomy. The device is unsheathed by withdrawing the access sheath until its distal portion forms a ball and the system can be carefully advanced or withdrawn within the LAA to achieve optimal positioning (within the landing zone). At this point, the delivery cable is advanced while further withdrawing the access sheath to uncover the device disc (“push and pull”). Before release, it's important to confirm the following:
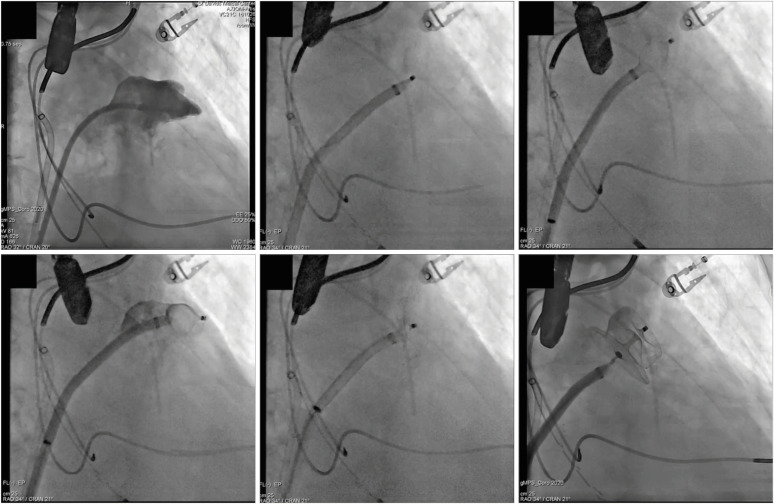 | Figure 6 Amulet deployment. Step-by-step procedure as seen on fluoroscopy. See text for description. Reproduced with permission from Gianni et al.19)

|
• Proper positioning: the lobe should be perpendicular to the LAA neck (coaxial to the LAA axis), lying beside the circumflex artery (two thirds of its length distal to it)
• Stability: assessed with a tug test (see above)
• Correct size: the device widest diameter on TEE should be compressed 2 to 4 mm of its original size; the lobe should look tire-shaped on fluoroscopy
• Proper occlusion: the disc should be adequately separated from the lobe with a concave shape, with no peri-device flow seen on contrast injection/color Doppler
If the device positioning is unsatisfactory, the disc and lobe can be sheathed back into the “ball” configuration, paying attention not to cover the 2 radiopaque markers on the device (location of the stabilizing wires/hooks) do not enter the radiopaque marker band on the sheath. If the size is inadequate or the markers enter beyond the radiopaque marker band, the device must be entirely removed, and the sheath replaced. Once LAAO is satisfactory, the device can be released by unscrewing the connector wire.
Go to :

WAVECREST
The WaveCrest (Biosense Webster, Diamond Bar, CA, USA) LAAO device is a single lobe device that consists of a self-expanding nitinol frame covered by a polytetrafluoroethylene (ePTFE; also known as Gore-Tex) knit fabric (
Figure 1). The ePTFE membrane covers the proximal surface, including the proximal screw end, to prevent thrombosis on the portion of the device in contact with the systemic circulation. A rim of polyurethane is located at the end of the membrane, where the device is in contact with the myocardium, to promote endothelialization. At the distal end, the frame perimeter is provided with 20 fixation hooks to anchor the device to the LAA and ensure its stability (as opposed to the other devices, WaveCrest has no radial force to aid with stability). Unique to the WaveCrest, the hooks are retractable, which separates positioning of the device from its anchoring. The device is packaged in a pre-loaded delivery sheath and is placed in the LAA by means of a 12-F access sheath. The access sheaths are provided in 4 shapes, which differs in the angle of the curve and orientation of the distal curve: single curve (60°, 75°, and 90°), and anterior curve (90°).To confirm good sealing, contrast can be injected through both sheaths: injection through the delivery sheath, distal to the device, is unique to WaveCrest and allows for leak detection by means of contrast extravasation. The device is manufactured in 3 sizes (22, 27, and 32 mm), which accommodate most LAA dimensions. The length of the device is less than 10 mm, which makes it attractive for the smallest LAA anatomies. As with Watchman, selection of the access sheath is usually physician preference, although the anterior curve is preferable for LAAs with a more superior direction (toward the aortic root).
WaveCrest device sizing is based on (
Figure 2):
• LAA landing zone width, measured from the circumflex artery to the transition between the smooth LA and the trabeculated LAA (usually 1 to 2 cm distal to the limbus of the LAA-left superior pulmonary vein ridge; same as Watchman): between 15 and 29 mm
• LAA depth plays a minor role as the level of the landing zone marks the distal device margin (i.e., should be more than 10 mm)
An oversizing of 3 to 8 mm is usually recommended for improved device stability. As with other LAAO devices, excessive oversizing should be avoided as it may result in device embolization.
For WaveCrest deployment (
Figure 7), the access sheath is carefully advanced over a pigtail catheter until the appropriate radiopaque marker band (according to the device size) lies at the level of landing zone. This is best done using a RAO caudal projection, but different views might be used according to the specific anatomy. The device is fully unsheathed by withdrawing the access sheath and the system can be carefully manipulated within the LAA to achieve optimal positioning (within the landing zone). At this point, the anchoring hooks are deployed for fixation. A radiopaque marker is visible at the level of the hooks and facilitates fluoroscopic assessment of their position. Before release, ultrasound/fluoroscopy are used to confirm:
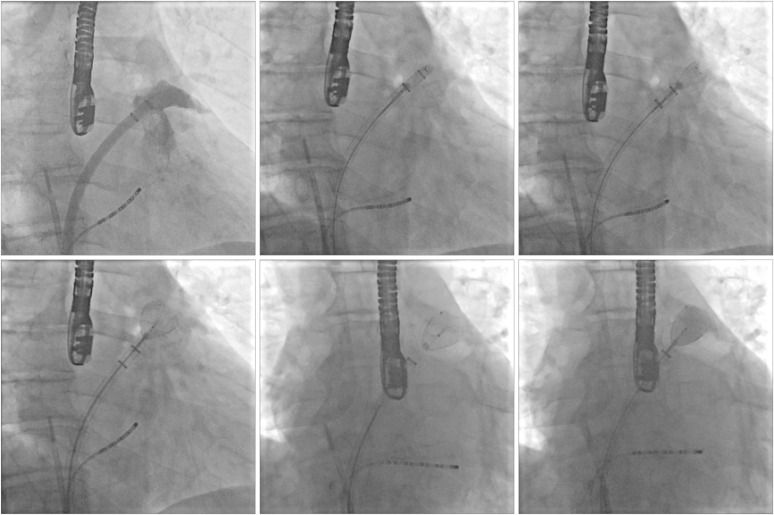 | Figure 7 WaveCrest deployment. Step-by-step procedure as seen on fluoroscopy. See text for description. Reproduced with permission from Gianni et al.19)

|
• Proper positioning: the lobe should lie within the proximal LAA
• Stability: assessed with a tug test (see above)
• Correct size: the device widest diameter should be compressed 3 to 8 mm of its original size
• Proper occlusion: no peri-device flow seen on proximal contrast injection/color Doppler, with no contrast extravasation with distal contrast injection
If the device positioning is unsatisfactory, the hooks can be retracted, and the device fully recaptured to be repositioned in a better location. Once LAAO is satisfactory, the device can be released by unscrewing the connector wire.
Go to :

LAMBRE
The LAmbre (Lifetech Scientific, Shenzhen, China) is a nitinol LAAO device conformed into a distal lobe and a proximal disc, both covered by a PET membrane, and connected by a short, central waist, thus providing a double sealing system (
Figure 1). The proximal disc is a nitinol mesh with a recessed proximal end screw to minimize the risk of device-related thrombosis, while the distal lobe is composed of a nitinol frame with 2 rows of 8 perimetral anchors to ensure its stability, which is aided by the mild radial force exerted by the frame. Unique to LAmbre, the articulated connecting waist which allows the disc and lobe to lie at different angles without affecting the device stability. The device is packaged in a pre-loaded delivery sheath and is placed in the LAA by means of an 8- to 10-F access sheath. There are 2 different type of manufactured LAmbre devices designed to accommodate single- and double-lobe anatomies. Each type comes in different sizes: 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, and 36 mm for the single-lobe design, and 16, 18, 20, 22, 24, and 26 mm for the double-lobe design. The disc diameter is 4 to 6 mm greater than the distal lobe in the single-lobe design, and 12 to 14 mm greater in the double-lobe design. As with WaveCrest, the length of the device is less than 10 mm, accommodating shallower LAAs. The access sheaths are provided in 2 shapes: single curve and double curve, chosen according to the operator preference.
LAmbre device sizing varies according to the design. On single-lobe devices, sizing is based on (
Figure 2):
• LAA landing zone width, measured from the circumflex artery to the transition between the smooth LA and the trabeculated LAA (usually 1 to 2 cm distal to the limbus of the LAA-left superior pulmonary vein ridge; same as Watchman and WaveCrest): between 11 and 28 mm
• LAA depth plays a minor role as the level of the landing zone marks the distal device margin (i.e., should be more than 10 mm)
On dual-lobe design devices, sizing is based on:
• LAA lobe landing zone width, measured around 1 cm distal to LAA bifurcation: between 11 and 20 mm
• LAA bifurcation depth, measured from the LAA ostial plane to the LAA lobe opening plane: less than 10 mm
• LAA lobe depth plays a minor role as the level of the landing zone marks the distal device margin (i.e. should be more than 10 mm)
An oversizing of 3 to 8 mm is usually recommended for improved device stability. As with other LAAO devices, excessive oversizing should be avoided as it may result in device embolization.
For LAmbre deployment, the access sheath is carefully advanced over a pigtail catheter until distal tip lies at proximal end of the LAA. This is best done using a RAO caudal projection, but different views might be used according to the specific anatomy. The distal lobe is unsheathed by pushing it forward to the landing zone, where the anchoring hooks can grasp the LAA, stabilizing the device. At this point, the delivery sheath is slowly withdrawn to expose the disc, which covers the LAA. Before release, ultrasound/TEE are used to confirm:
• Proper positioning: the lobe should lie within the proximal LAA/proximal aspect of the chosen lobe
• Stability: assessed with a tug test (see above)
• Correct size: the device widest diameter should be compressed 3 to 8 mm of its original size
• Proper occlusion: the disc should be adequately separated from the lobe with a concave shape, with no peri-device flow seen on contrast injection/color Doppler
If the device positioning is unsatisfactory, the device can be fully recaptured and repositioned in a better location. Once LAAO is satisfactory, the device can be released by unscrewing the connector wire.
Go to :

LARIAT
The Lariat (SentreHeart, Redwood City, CA, USA) LAAO device is a suture-based device that allows epicardial LAA ligation with no device left in the endocardial space. The non-absorbable size 0 braided polyester suture is pre-tied and loaded on a 12-F suture delivery device consisting of a collapsible 40- or 45-mm snare (
Figure 1). To perform epicardial ligation, there are other four necessary components: a 9-F 15-mm diameter by 12-mm length compliant occlusion balloon catheter, two magnet-tipped guidewires (0.025″ and 0.035″), a suture tightener, and a suture cutter.
Imaging is used to confirm that the LAA anatomy is suitable for epicardial closure:
• LAA ostial diameter: less than 45 mm (not exceeding the snare diameter)
• LAA depth: less than 70 mm
• LAA position: it should not be posteriorly oriented LAA with its apex behind the pulmonary artery
Given the need for epicardial instrumentation, patients with an history of prior cardiac surgery, pericarditis, or radiation to the chest are not candidates for Lariat LAAO because pre-existing adhesions preclude pericardial access. As opposed to other intravascular devices, the procedure should be performed off therapeutic anticoagulation. It is also reasonable to prescribe a short course of colchicine (to be started 2–3 days before the procedure) to reduce the incidence of post-procedural pericarditis.
24)25)
Lariat LAAO requires an endo-epicardial approach (
Figure 8) and the deployment requires three hands: thus, two operators are necessary, the primary operator with either an assistant or an experienced scrub technician. The first step of a Lariat procedure is obtaining anterior epicardial access: this is achieved by using fluoroscopy in a LL view, to confirm guidewire advancement to the right ventricle. For epicardial access, a 17-gauge blunt-tip epicardial needle (Tuohy) or a micropuncture set are preferred to minimize the risk of coronary/myocardial injury. Following successful epicardial access, transeptal access is obtained and the endocardial balloon/0.025″ magnet-tipped guidewire assembly is positioned in the left atrium. The tip of the endocardial guidewire is then positioned at the apex of the LAA and the 0.035″ epicardial magnet-tipped guidewire is advances towards the LAA until they magnetically bind to each other. The endocardial balloon is inflated with a mix of 1:1 saline and contrast the advanced over the endocardial guidewire at the ostium of the LAA. The suture delivery device is advanced over the epicardial guidewire until the snare is positioned over the ostium of the LAA, which is designed by the radiopaque marker proximal to the inflated balloon. The snare is then closed and proper LAAO (i.e., absence of leaks) is confirmed by ultrasound/fluoroscopy. If necessary, the snare can be re-opened the device positioned in a different location. Once LAAO is satisfactory, the endocardial balloon/magnet-tipped guidewire assembly is withdrawn from the LAA and the suture is deployed from the snare using the dedicated suture tightener (2 applications, 5 minutes apart) and suture cutter.
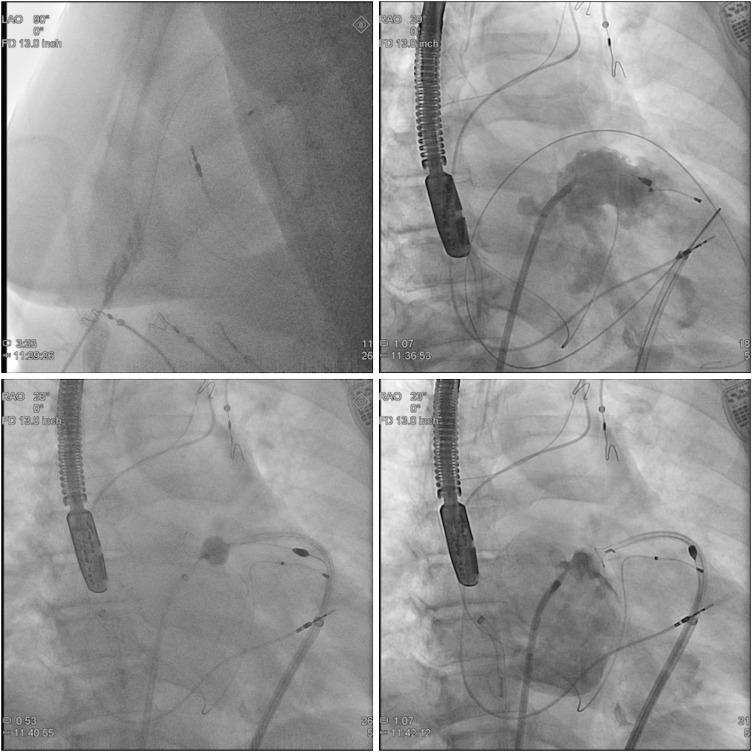 | Figure 8 Lariat deployment. Step-by-step procedure as seen on fluoroscopy. See text for description.
|
At the end of the procedure, a pericardial drain is usually left in place overnight and a liposomal bupivacaine (half-life more than 24 hours)/steroid solution can be applied epicardially to further mitigate the pain/inflammatory response post-LAA ligation.
26)
Go to :












 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download