INTRODUCTION
Portal hypertension, which is defined as an increase in the pressure within the portal vein, often develops in the setting of liver cirrhosis. Increased portal pressure may lead to the development of varices and even variceal hemorrhage.
1 Varices are typically located in the gastroesophageal region but can occur in the ectopic regions. Ectopic varices account for up to 5% of all variceal bleeding,
2 and 17% of them are located in the duodenum.
3 Bleeding from the duodenal varices is rare, but bleeding can be massive and sometimes fatal.
4 Several therapeutic modalities are used for duodenal variceal bleeding, including endoscopic sclerotherapy, endoscopic variceal ligation, transjugular intrahepatic portosystemic shunt (TIPS), and balloon-assisted retrograde transvenous obliteration (BRTO). On the other hand, the optimal treatment modality has not been established. This case report introduces a patient with duodenal variceal bleeding who was managed successfully using percutaneous trans-splenic variceal obliteration (PTVO).
Go to :

CASE REPORT
A 56-year-old man visited the emergency department reporting a 6-day history of melena. He disclosed a significant amount of alcohol intake (150 g daily) for more than 30 years. He had been diagnosed with alcoholic cirrhosis seven years ago but did not undergo a regular follow-up. At the time of presentation, his blood pressure was 140/90 mmHg, and his pulse was 97 beats/min. He appeared acutely ill. The physical examination revealed pale conjunctiva, and the digital rectal examination was positive for dark blood. The initial laboratory evaluation revealed a leukocyte count of 5,120/mm3, a hemoglobin level of 7.9 g/dL, and a platelet count of 141,000/mm3. His serum bilirubin level was 1.1 mg/dL, with an albumin level of 2.5 g/dL, PT-international normalized ratio of 1.15, ALP level of 363 U/L, AST level of 71 U/L, and ALT level of 18 U/L. The serology tests were negative for HBsAg, anti-HBs, and anti-HCV. The serum AFP level was 4.07 ng/mL.
Emergency esophagogastroduodenoscopy (EGD) was performed on the day of admission (
Fig. 1A). No focus of bleeding was identified, nor was there evidence of bleeding in the esophagus or stomach. On the other hand, a large varix was present in the second portion of the duodenum. No active bleeding was observed, but a nipple sign was noted, indicating recent bleeding. Dynamic CT was performed to visualize duodenal varix and the feeding veins and determine if a therapeutic radiologic intervention, such as TIPS or BRTO, could be undertaken (
Fig. 1B, C). Dynamic CT revealed a cirrhotic liver with splenomegaly and duodenal varix. A focal, early-enhancing nodular lesion was noted in segment 5 of the liver, which suggested a hepatocellular carcinoma (HCC) (
Fig. 1D-F). The duodenal varix was formed by a mesocaval shunt that is supplied with blood by the pancreaticoduodenal vein and drained into the inferior vena cava. A retrograde transvenous approach to the varix was deemed too difficult because of the acute angle of the efferent vein. Therefore, TIPS or BRTO were not conducted, and PTVO was performed instead with the intent of obliterating the duodenal varix by coil embolization via a percutaneous trans-splenic approach. The catheter was introduced by trans-splenic puncture under ultrasound- and fluoroscopic guidance, inserted into the splenic vein, and advanced to the portal vein. Direct portography revealed the shunt between the afferent feeding vein, originating from the portal vein, and duodenal varix (
Fig. 2A). Coil embolization was performed using a detachable coil and a 1:1 mixture of N-2-butyl cyanoacrylate (NBCA) and ethiodized oil (Lipiodol
® Ultra Fluid, Guerbet, Villepinte, France) at the duodenal varix and afferent feeding vein. Complete obliteration was confirmed at the following portography (
Fig. 2B, C). To prevent bleeding from the trans-splenic puncture, tract embolization was also performed with a 0.035 inch Nester
® Embolization Coil (Cook Medical, Bloomington, IN, U.S.A.) and a 1:1 ratio of NBCA/Lipiodol mixture at the puncture site.
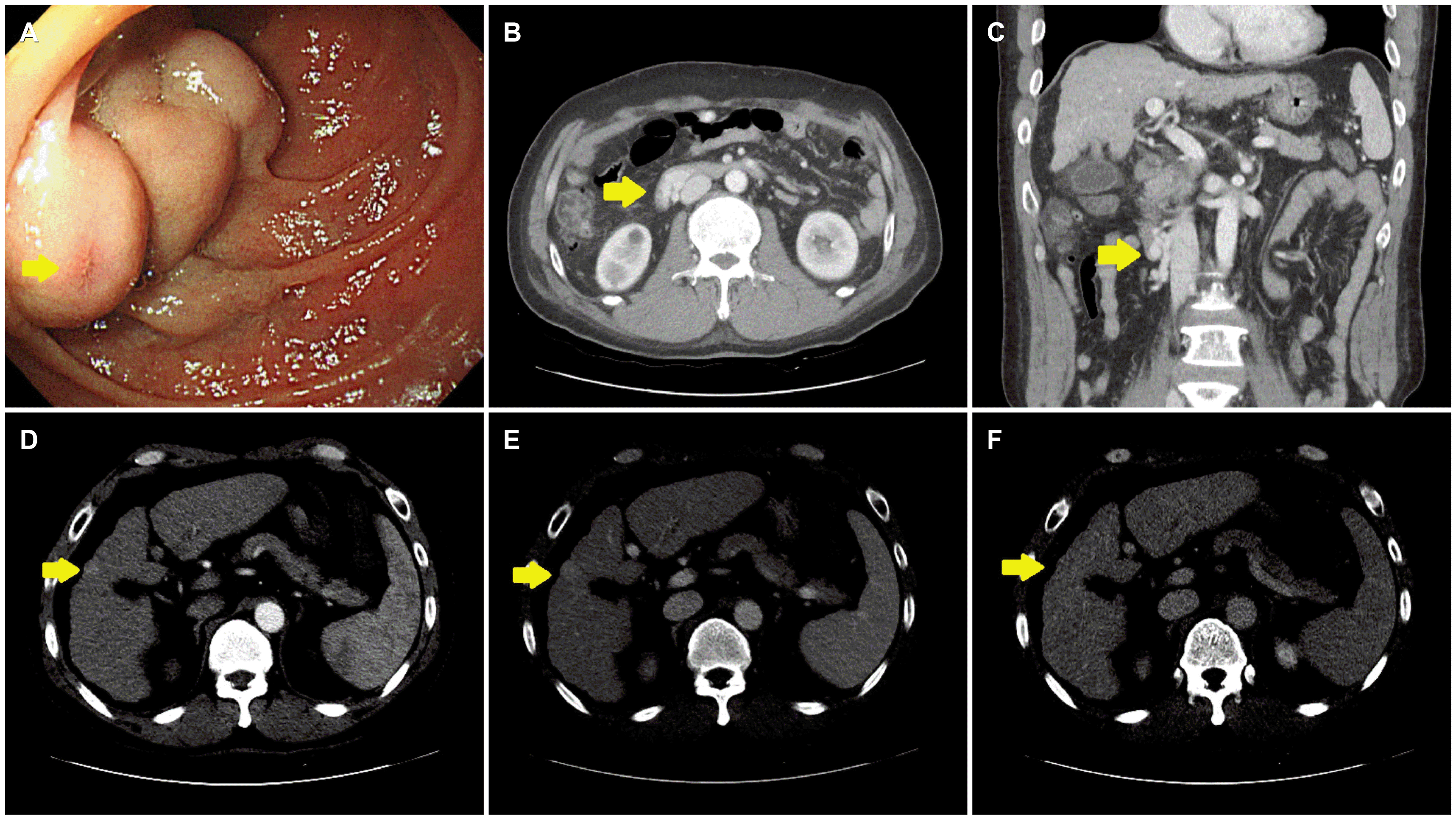 | Fig. 1Esophagogastroduodenoscopy (EGD) and dynamic computed tomography (CT) at the time of initial diagnosis. (A) EGD reveals a large varix measuring >5 mm with a nipple sign (arrow) in the second portion of the duodenum. (B, C) The portal phase of dynamic CT shows a varix in the second portion of the duodenum (arrows). (D) A hepatic nodule in segment 5 (arrows) measures about 1 cm and exhibits enhancement in the arterial phase (E) without washout in the portal phase or (F) delayed phase. 
|
 | Fig. 2Percutaneous trans-splenic variceal obliteration for the treatment of duodenal variceal bleeding. (A) Direct portography shows a shunt between the afferent feeding vein, originating from the portal vein, and the duodenal varix. (B) Coil embolizationis successfully performed. (C) Complete obliteration is confirmed in the following portography. 
|
The patient’s vital signs remained stable after treatment, and he no longer complained of melena. Three days later, follow-up EGD and imaging were performed to verify the obliteration of the duodenal varix. The follow-up EGD revealed a significant decrease in the size of the duodenal varix, and the nipple sign was not observed (
Fig. 3A). Follow-up dynamic CT showed a persistent duodenal varix but with a reduced size (
Fig. 3B, C). A nonselective beta-blocker was prescribed to reduce portal pressure, and the patient was discharged 4 days after PTVO.
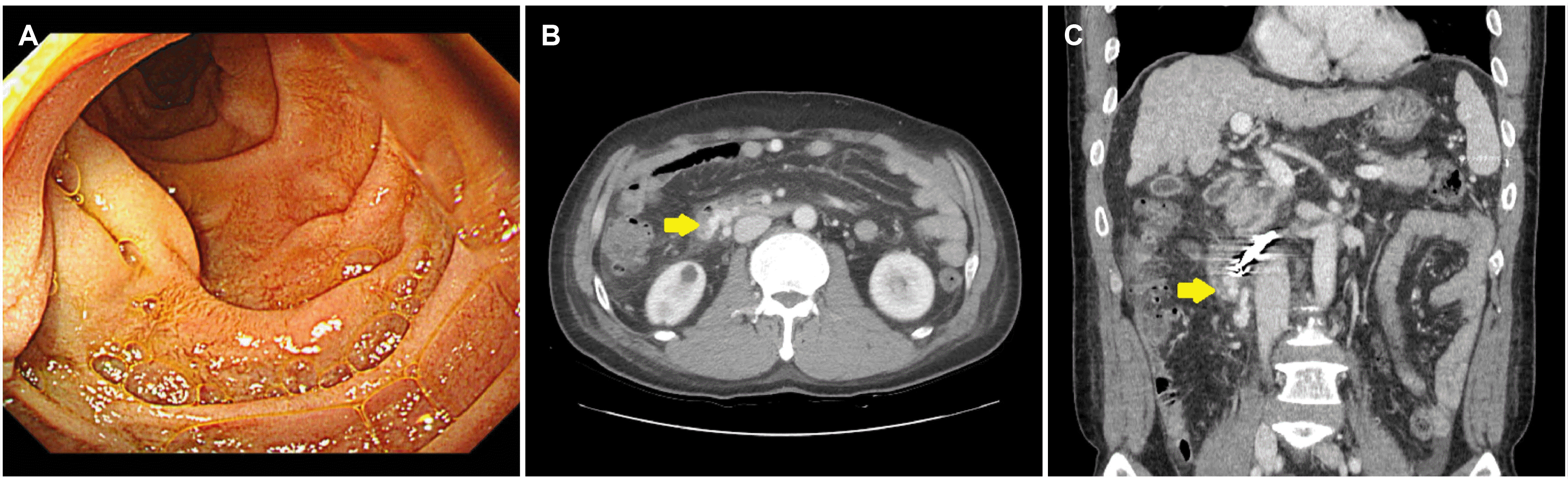 | Fig. 3Esophagogastroduodenoscopy (EGD) and dynamic computed tomography (CT) performed 3 days after percutaneous trans-splenic variceal obliteration. (A) EGD reveals a varix in the second portion of the duodenum that decreased in size from the initial examination (B, C) The portal phase of dynamic CT shows a duodenal varix decreased in size from the time of initial imaging (arrows). 
|
The patient attended the follow-up visits at the outpatient clinic every 2 to 4 weeks and no longer complained of melena. Three months after PTVO, repeated EGD and dynamic CT were performed to verify the status of the duodenal varix and investigate the previously noted suspicious hepatic nodule. The varix was no longer visible on EGD (
Fig. 4A), and dynamic CT showed that the duodenal varix was still present but had decreased further in size, without the development of additional ectopic varices (
Fig. 4B, C). The hepatic nodule that was suspicious for HCC had not changed in size, and other intrahepatic lesions were not detected (
Fig. 4D-F). The patient’s hemoglobin level and serum AFP level were 10.7 g/dL and 3.83 ng/mL, respectively. The patient was followed regularly at the outpatient clinic for more than 10 months after the PTVO procedure; he had no further complaints of melena.
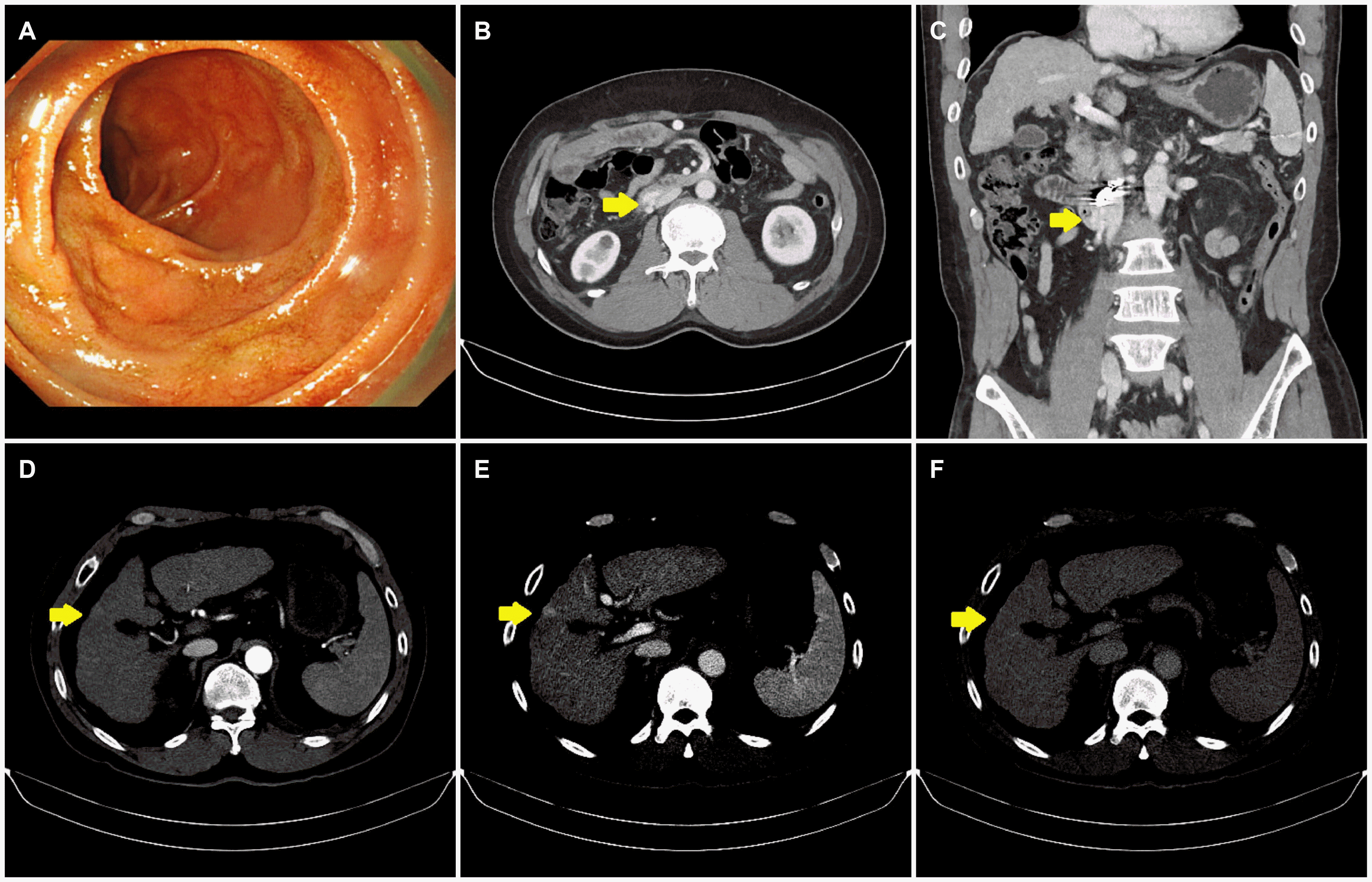 | Fig. 4Esophagogastroduodenoscopy (EGD) and dynamic computed tomography (CT) performed 3 months after percutaneous trans-splenic variceal obliteration. (A) EGD reveals that the varix in the second portion of the duodenum are no longer visible. (B, C) The portal phase of dynamic CT shows that the size of the duodenal varix (arrows) have decreased in size compared with the imaging performed 3 days after treatment. (D) The hepatic nodule in segment 5 (arrows) exhibits faint enhancement in the arterial phase (E) without washout in the portal phase or (F) delayed phase. It has not changed in size compared with the imaging obtained at the time of diagnosis. 
|
Go to :

DISCUSSION
Liver cirrhosis is defined by histologic changes representing regenerative nodules surrounded by fibrous tissue. These changes occur in response to chronic liver damage, such as viral hepatitis or chronic alcoholism. These pathologic changes increase the intrahepatic vascular resistance, eventually leading to portal hypertension. The major complications of portal hypertension include ascites and gastroesophageal varices. Although the incidence is very low, duodenal varices can also develop, with or without gastroesophageal varices. Duodenal variceal bleeding has a reported mortality of 40% due to the massive and recurrent nature of the bleeding.
3 Unfortunately, although several therapeutic modalities have been attempted, the optimal treatment of duodenal variceal bleeding is still unclear because of its rarity.
Endoscopic interventions for duodenal variceal bleeding, including variceal ligation and sclerotherapy, are relatively non-invasive and can induce hemostasis at the time of diagnosis. Hence, they are useful for patients with active bleeding and unstable vital signs. On the other hand, visualization of the duodenal varices may be difficult if the varices are located in the distal duodenum. Furthermore, endoscopic variceal ligation can cause a wider defect and have been reported to carry a high risk of rebleeding.
5 Endoscopic sclerotherapy also can cause complications, including tissue damage, ulceration, and perforation, and it can increase the risk of pulmonary embolism.
6 EUS is a useful modality for not only an evaluation but also a treatment of duodenal varices. A few case reports have revealed the successful management of duodenal variceal bleeding using EUS-guided placement of a coil along with an NBCA injection.
7,8 Nevertheless, the EUS-guided therapy may be problematic in patients with duodenal variceal bleeding because the manipulation of the endoscope inside the duodenum and visualization of the varices are difficult during active bleeding. Such endoscopic interventions were not performed in the present patient because there was no active bleeding from the large duodenal varix, and his vital signs were stable.
Radiologic interventions, including TIPS and BRTO, can be used for the treatment of duodenal variceal bleeding. The use of TIPS is safe and effective; it decreases the portosystemic pressure gradient immediately.
9 TIPS, however, has contraindications, including incipient hepatic encephalopathy, decompensated liver disease, hepatoma, and unfavorable venous anatomy.
10 In contrast to TIPS, the use of BRTO can completely embolize the targeted varices without reducing the portal flow. Therefore, it can be performed even in patients with decreased liver function.
11 On the other hand, BRTO is difficult to implement in patients with an unfavorable venous anatomy, making it challenging to advance a balloon catheter to the target varices. TIPS or BRTO were not considered in the present patient because of the focal, enhancing hepatic nodule that was suspicious of HCC and because of the unfavorable venous anatomy.
Percutaneous variceal obliteration can also be a viable option for patients with duodenal variceal bleeding. The targeted varices can be treated through the percutaneous approach, not through the transvenous approach, and allows for embolization. Therefore, percutaneous obliteration can control hemorrhage immediately by directly approaching the targeted varices, even in patients with an unfavorable vascular anatomy. Furthermore, percutaneous obliteration reportedly improves the hepatic function reserve by increasing the antegrade blood flow to the liver.
12 On the other hand, the risk of rebleeding was reported to be high after emergency percutaneous obliteration for variceal hemorrhage.
13,14 Therefore, portal decompression therapies, such as the use of beta-blockers or management of the underlying liver disease, should be employed after emergency percutaneous variceal obliteration. Several reports have described the transhepatic approach to percutaneous obliteration to treat duodenal variceal bleeding without complications successfully.
15,16 Despite this, there are relatively few reports of percutaneous obliteration using a trans-splenic approach (i.e., PTVO) for this indication.
17,18 In addition, unlike previous studies, this study used NBCA glue and an embolization coil to facilitate a clotting process in the varices and reduce the amount of NBCA injection required.
Several reports have shown that the percutaneous trans-splenic approach is a safe and effective method to assess the portal vein.
19,20 This can also be used in patients whose portal vein cannot be assessed through the transhepatic approach because of the portal vein thrombosis. On the other hand, the trans-splenic approach is not the first choice to treat variceal bleeding when the transhepatic approach is avail-able because it may have higher bleeding or mortality rates than the transhepatic approach.
20 The trans-splenic approach can lead to splenic puncture tract bleeding and/or intrasplenic hematomas, particularly in patients with portal hypertension, splenomegaly, and thrombocytopenia.
19 Therefore, tract embo-lization was performed using a coil and NBCA glue to prevent bleeding complications. The patient was successfully treated for duodenal variceal bleeding without any bleeding complications. Ten months after the procedure, there has been no further gastrointestinal bleeding.
In conclusion, this paper described a patient with duodenal variceal bleeding who was managed successfully using PTVO. Overall, PTVO may be a useful treatment option for patients with duodenal variceal bleeding in whom endoscopic inter-ventions or conventional radiology interventions, such as TIPS or BRTO, are difficult.
Go to :









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download