Abstract
Low-grade endometrial stromal sarcoma (LG-ESS) is a very rare mesenchymal neoplasm of the uterus. LG-ESS can recur or metastasize to extrauterine sites, such as the pelvis, peritoneal cavity, and vagina, but rarely to the lung, liver, heart, bone, and colon. A 42-year-old female patient was transferred from an outside clinic for an evaluation of constipation. EUS revealed a 5 cm hypoechoic lesion with a regular margin, probably arising from the 4th layer (muscular propria) at the sigmoid colon level. CT revealed a 7-cm homogenous enhancing mass lesion at the pelvic cavity and multiple enlarged lymph nodes in the sigmoid mesocolon. The patient underwent an anterior resection, and the diagnosis based on the biopsy result was LG-ESS. After a multidisciplinary discussion, she underwent a bilateral salpingo-oophorectomy. Small nodules found in the endometrium were identified as LG-ESS by a biopsy. This paper reports a case of metastatic LG-ESS presenting as a solitary sigmoid tumor without intrauterine lesions through preoperative examinations and discusses the characteristics of this neoplasm with reference to the relevant literature.
Go to : 
Low-grade endometrial stromal sarcoma (LG-ESS) is a very rare mesenchymal neoplasm of the uterus, accounting for 0.2% of all uterine malignancies. The disease often has low-grade, slow-growing, and indolent features. On the other hand, metastasis or recurrence can occur occasionally after a long period. LG-ESS can recur or frequently metastasize to extrauterine sites, such as the pelvis, peritoneal cavity, and vagina, but rarely to the lung, liver, heart, bone, and colon.1-6 On the other hand, a diagnosis of extrauterine LG-ESS in the absence of intrauterine lesions in preoperative examinations is difficult. This paper reports a case of metastatic LG-ESS presenting as a solitary sigmoid tumor without intrauterine lesions in preoperative examinations and discusses the characteristics of this neoplasm with reference to the literature.
Go to : 
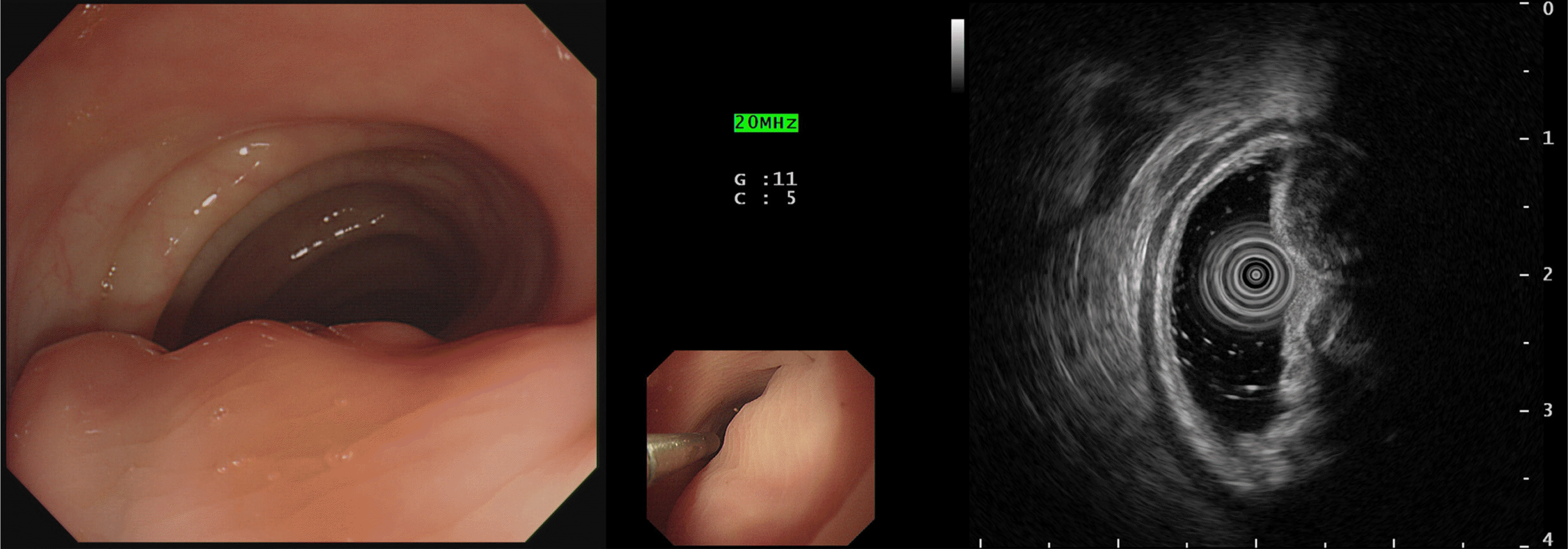
The Institutional Review Board of Wonkwang University Hospital (WKUH 2019-08-010) approved this study, and informed consent was waived. A 42-year-old female patient was transferred from an outside clinic for constipation that had lasted for 2 months. In a previous sigmoidoscopy performed at an outside clinic, an approximately 5-cm subepithelial tumor was detected in the sigmoid colon. She had a medical history of appendectomy and uterine myomectomy. The patient’s blood pressure, pulse rate, and body temperature were 110/70 mm/Hg, 70 beats/min, and 36.5℃, respectively. The patient’s laboratory test results were as follows: hemoglobin, 9.5 g/dL; white blood cell count, 4.52×103/mm3; carcinoembryonic antigen, 1.08 ng/mL. The other blood chemistry parameters and tumor markers were within the normal limits. She underwent EUS to evaluate the subepithelial tumor, which revealed a 5 cm hypoechoic lesion with a regular margin probably arising from the 4th layer (muscular propria) at the sigmoid colon level. A biopsy was not performed because of minimal changes in the mucosa (Fig. 1).
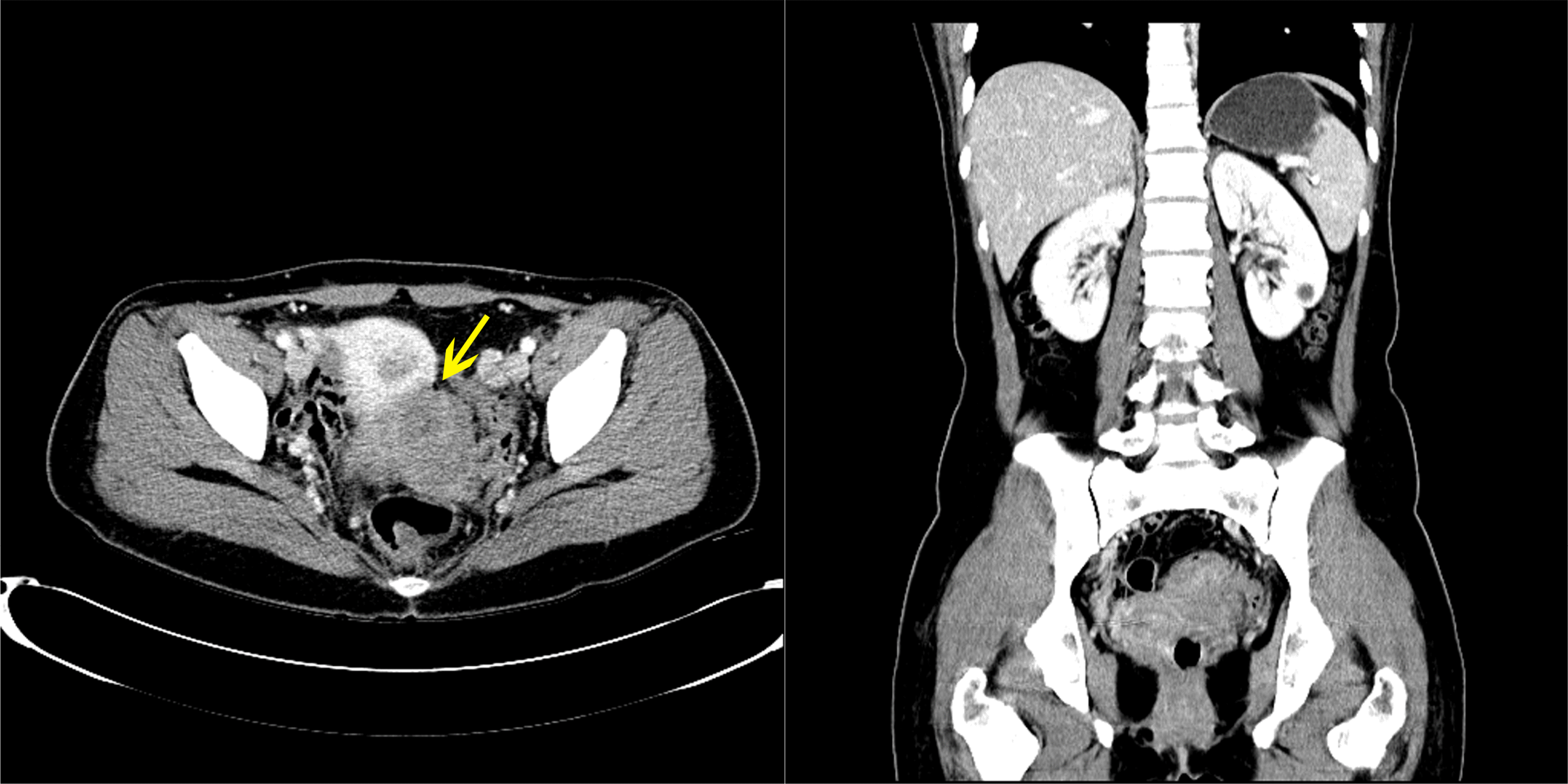
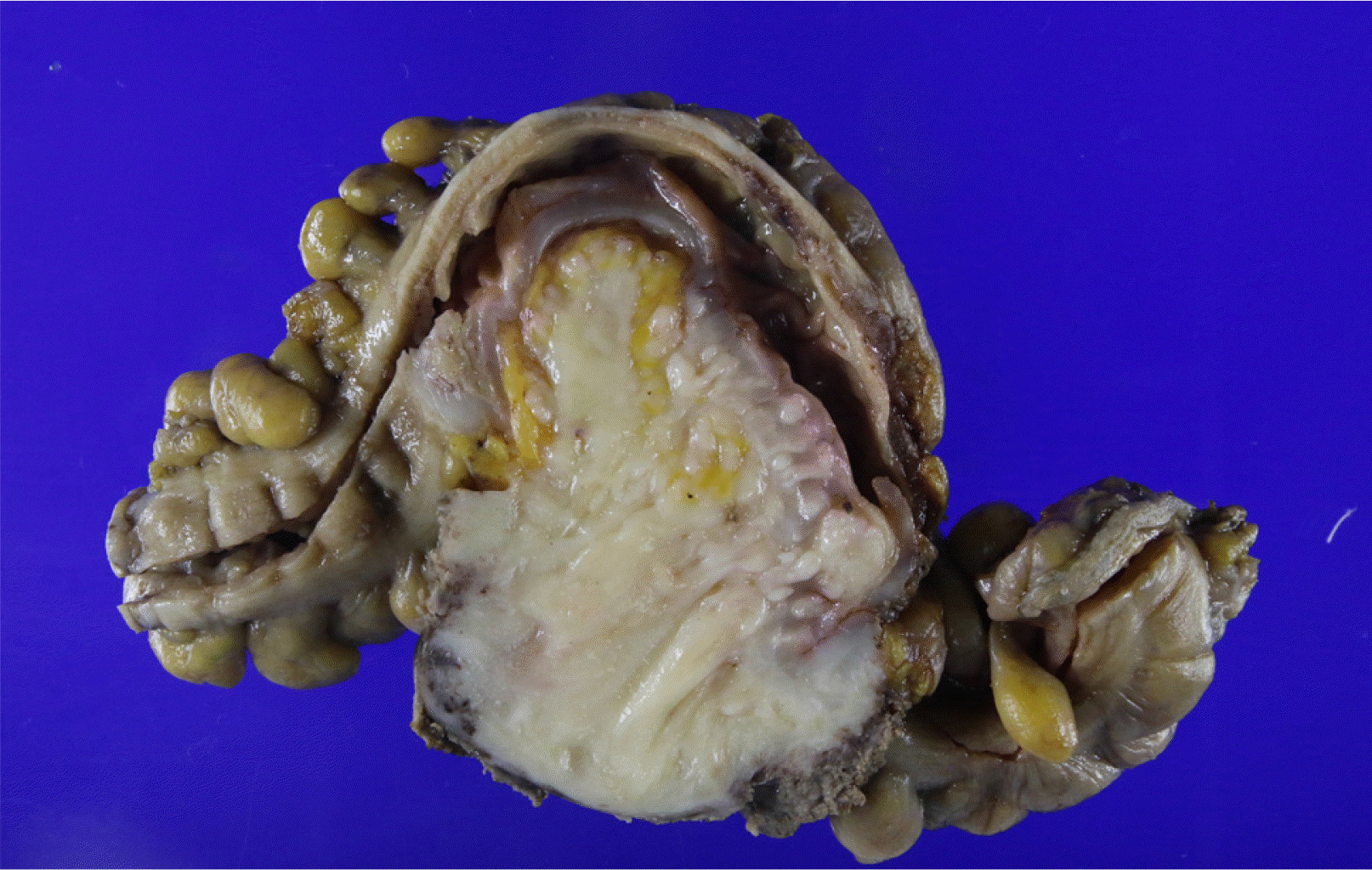
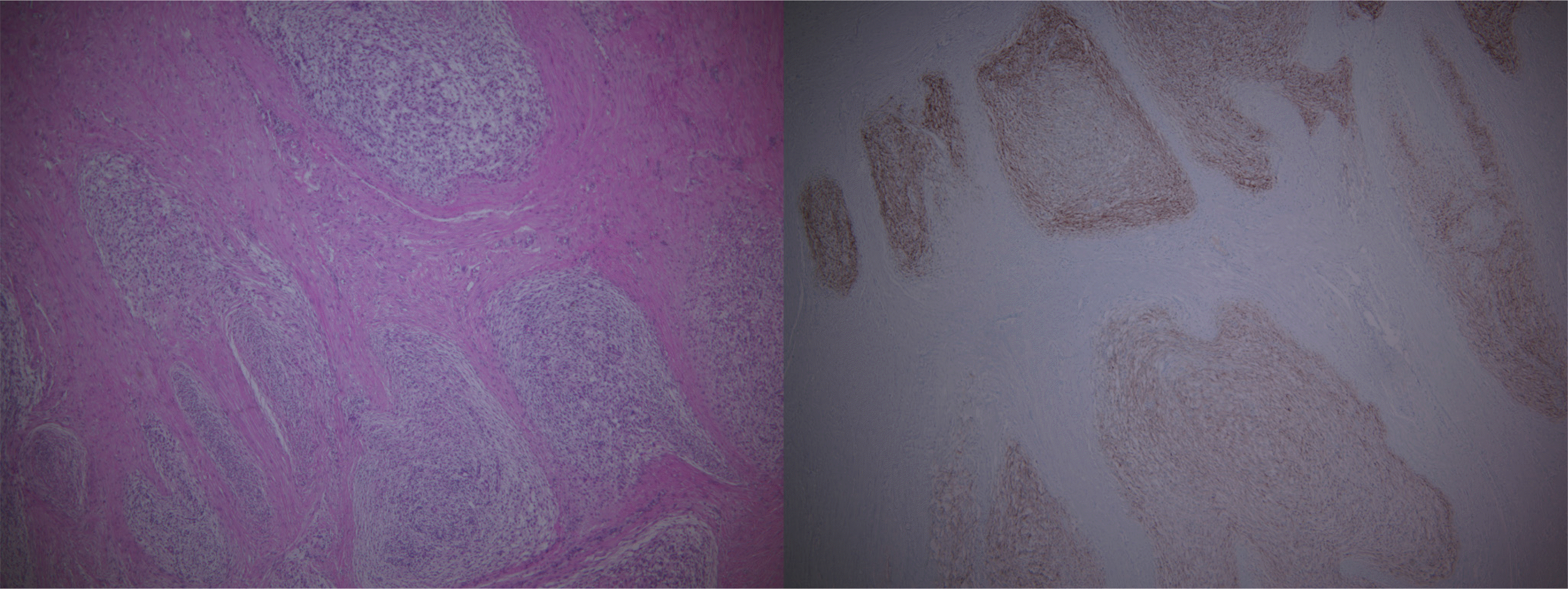
CT showed a 7-cm homogenous enhancing mass lesion at the pelvic cavity and multiple enlarged lymph nodes in the sigmoid mesocolon. The mass appeared to be a subepithelial lesion of the sigmoid colon, but its origin was unclear because it was adjacent to the cervix (Fig. 2). Therefore, a MRI scan was performed to confirm the origin of the tumor, and a gynecological consultation was performed. MRI showed that the tumor was separated from the cervix. On the other hand, it was still adjacent to the cervix, suggesting an invasion, and there were no unusual findings in the uterus, except for myoma degeneration. In January 2019, the patient underwent a laparoscopic anterior resection under the suspicion of a subepithelial tumor, such as gastrointestinal stromal tumors. The surgical findings revealed a 7×5 cm mass lesion at the sigmoid and multiple peritoneal nodules in the sigmoid mesocolon (Fig. 3). The main mass was adjacent to the cervix, but a dissection was successfully achieved. A frozen biopsy of the peritoneal nodule in the sigmoid mesocolon was conducted, and the result revealed endometriosis. A hysterectomy was not performed because there were no abnormal findings in the uterus. Pathologically, the mass showed a sheet of monomorphic spindle cells with scant cytoplasm and round or ovoid nuclei with dispersed chromatin. The mitotic features were infrequent. Immunochemical staining for CD10, estrogen receptor (ER), and progesterone receptor (PR) was strongly positive (Fig. 4). The tumor had not metastasized to the lymph node (0/10).
The diagnosis based on the biopsy result was LG-ESS. After diagnosis, PET/CT, gynecological consultation, and multidisciplinary discussion were conducted to confirm the extrauterine ESS by checking the intrauterine lesions. Although there were no abnormal findings in the PET/CT, a multidisciplinary discussion led to a hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO). Six months after the initial surgery, the patient underwent TAH-BSO. Small nodules were found in the endometrium, and the biopsy identified them as low-grade ESS. The final diagnosis confirmed that the metastatic ESS was a sigmoid tumor. The patient will continue to receive gynecological treatment, including hormone therapy after recovery.
Go to : 
LG-ESS is a rare mesenchymal tumor of the uterus that consists of cells resembling proliferative endometrial stromal tissue. Traditionally, malignant ESS had been divided into low- and high-grade groups according to the mitotic index. On the other hand, ESS is re-classified into four subgroups according to the 2014 World Health Organization classification, includ-ing endometrial stromal nodule (ESN), LG-ESS, high-grade ESS, and undifferentiated uterine sarcoma. ESN is a benign lesion, and its morphological, immunochemical, and molecular characteristics are similar to those of LG-ESS. Compared to ESN, LG-ESS can be distinguished by its invasion into the myometrium and exhibiting an infiltrative growth pattern. High-grade ESS involves a chromosomal translocation of YWHAE-FAM22, characterized by uniform but more atypical neoplastic cells. Undifferentiated uterine sarcoma is a highly malignant sarcoma that lacks endometrial stromal differentiation and presents a complex karyotype without specific translocation. Among them, LG-ESS is an indolent tumor with a better prognosis.7-9 On the other hand, approximately 50% of patients with LG-ESS show recurrent disease, and recurrences or metastases are detected after a long period. The common site of metastasis or recurrence is the vagina, pelvis, and peritoneal cavity, but rarely the lung, liver, heart, bone, and colon. In addition, it can seldom occur at the extrauterine site, including the gastrointestinal tract, without a primary lesion of the uterus associated with endometriosis.1,2,10
The patient did not complain of gynecological symptoms, including vaginal bleeding or pelvic pain, but merely visited the hospital for constipation. The extrauterine lesion was identified by colonoscopy first. Before surgery, this lesion was suspected to be a GIST based on the colonoscopy findings and imaging studies because there were no intrauterine lesions in the preoperative examinations. On the other hand, the biopsy result of the colon lesion confirmed it to be LG-ESS. TAH-BSO was performed after a multidisciplinary discussion. As a result, the lesion was finally confirmed to be a metastatic LG-ESS case through the subsequent biopsy results after TAH-BSO. Therefore, if extrauterine lesions are found first, a close evaluation of the uterus should be conducted to confirm the presence of a primary lesion because an extrauterine ESS is extremely rare. In addition, since LG-ESS tends to recur or metastasize, it is necessary to recheck the previous biopsy results to determine if a hysterectomy had been performed previously. Furthermore, in cases of LG-ESS occurring in the extrauterine site, particularly the gastrointestinal tract, it is necessary to distinguish it from other mesenchymal tumors. Most other mesenchymal tumors can be excluded based on their histological characteristics. On the other hand, they have the potential to be confused as GISTs. Immunohistochemical staining might help distinguish both tumors. Although GIST is positive for CD34 and CD117, LG ESS is positive for CD10, ER, and PR.3,10
The standard treatment for primary LG-ESS is a surgical resection. Some investigators have proposed surgical cytoreduction when extrauterine lesions are present. On the other hand, the role of chemotherapy and radiation therapy has not been established. Postoperative radiation therapy reduces local recurrence but does not improve overall survival.3,5,6,11,12 Hormone therapy can play an important role in the treatment of LG-ESS because this tumor is usually characterized by estrogen and progesterone receptors and is often sensitive to hormones. Adjuvant progesterone should be considered in LG-ESSs. Some studies revealed a prolonged complete response in patients with resected LG-ESS. On the other hand, tamoxifen and estrogen are contraindications because they have a stimulating effect on disseminated endometrial stromal cells.6,12,13
In conclusion, this paper reports a case of metastatic LG-ESS that presented as a solitary sigmoid mass without intrauterine lesions in the preoperative examinations. This case highlights the need for a close evaluation of primary lesions in the uterus if extrauterine LG-ESS is found.
Go to : 
REFERENCES
1. Asada Y, Isomoto H, Akama F, et al. 2005; Metastatic low-grade endometrial stromal sarcoma of the sigmoid colon three years after hysterectomy. World J Gastroenterol. 11:2367–2369. DOI: 10.3748/wjg.v11.i15.2367. PMID: 15818757. PMCID: PMC4305830.

2. Ayuso A, Fadare O, Khabele D. 2013; A case of extrauterine endometrial stromal sarcoma in the colon diagnosed three decades after hysterectomy for benign disease. Case Rep Obstet Gynecol. 2013:202458. DOI: 10.1155/2013/202458. PMID: 23710389. PMCID: PMC3655501.

3. Kim L, Choi SJ, Park IS, et al. 2008; Endometrial stromal sarcoma of the small bowel. Ann Diagn Pathol. 12:128–133. DOI: 10.1016/j.anndiagpath.2006.03.020. PMID: 18325474.
4. Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. 1990; Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol. 14:415–438. DOI: 10.1097/00000478-199005000-00002. PMID: 2327549.
5. Matsuura Y, Yasunaga K, Kuroki H, Inagaki H, Kashimura M. 2004; Low-grade endometrial stromal sarcoma recurring with multiple bone and lung metastases: report of a case. Gynecol Oncol. 92:995–998. DOI: 10.1016/j.ygyno.2003.11.047. PMID: 14984975.

6. Bakker IS, Hoven-Gondrie ML, Moll FC, de Haan HH. 2013; A very late recurrence of a formerly misdiagnosed low grade endometrial stromal sarcoma metastasized to the colon. Int J Surg Case Rep. 4:1113–1116. DOI: 10.1016/j.ijscr.2013.09.017. PMID: 24240082. PMCID: PMC3860033.

7. Conklin CM, Longacre TA. 2014; Endometrial stromal tumors: the new WHO classification. Adv Anat Pathol. 21:383–393. DOI: 10.1097/PAP.0000000000000046. PMID: 25299308.
8. Kurman RJ, Carcangiu ML, Herrington CS, Young RH. 2014. WHO classification of tumours of female reproductive organs. 4th ed. IARC Press;Lyon:
9. Lee CH, Mariño-Enriquez A, Ou W, et al. 2012; The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 36:641–653. DOI: 10.1097/PAS.0b013e31824a7b1a. PMID: 22456610.
10. Son HJ, Kim JH, Kang DW, Lee HK, Park MJ, Lee SY. 2015; Primary extrauterine endometrial stromal sarcoma in the sigmoid colon. Ann Coloproctol. 31:68–73. DOI: 10.3393/ac.2015.31.2.68. PMID: 25960975. PMCID: PMC4422990.

11. Geas FL, Tewari DS, Rutgers JK, Tewari KS, Berman ML. 2004; Surgical cytoreduction and hormone therapy of an advanced endometrial stromal sarcoma of the ovary. Obstet Gynecol. 103:1051–1054. DOI: 10.1097/01.AOG.0000127942.91516.a2. PMID: 15121605.

12. Li N, Wu LY, Zhang HT, An JS, Li XG, Ma SK. 2008; Treatment options in stage I endometrial stromal sarcoma: a retrospective analysis of 53 cases. Gynecol Oncol. 108:306–311. DOI: 10.1016/j.ygyno.2007.10.023. PMID: 18061249.

13. Chu MC, Mor G, Lim C, Zheng W, Parkash V, Schwartz PE. 2003; Low-grade endometrial stromal sarcoma: hormonal aspects. Gynecol Oncol. 90:170–176. DOI: 10.1016/S0090-8258(03)00258-0.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download