INTRODUCTION
Probiotics are living microorganisms that benefit the host when administered in sufficient quantities.
1 Hundreds of probiotics species live symbiotically in the human colon, including bacteria, fungi, and protists.
Lactobacillus and
Bifidobacterium are representative, while others include
Bacillus and
Escherichia coli, and the yeast,
Saccharomyces boulardii.
2 The mechanism of action of probiotics is as follows: multiplying in the intestine first, then taking its place, and preventing pathogenic microorganisms from growing. The immune mechanism for this involves the activation of macrophages in the surrounding tissues, regulation of cytokines, and increasing tolerance for antigens in food. Non-immune mechanisms include consuming food substances required by pathogens, adjusting the pH in the intestine, removing superoxide radicals, increasing mucin secretion, and strengthening the intestinal barrier. Several studies are still ongoing, and due to these mechanisms, they are effective against acute diarrhea, anti-biotics-associated diarrhea, and diarrhea caused by
Clostridium difficile.
3 Many probiotics have already been commercialized, and further studies are being conducted on the dosage, individual species characteristics, and various effects in different diseases.
The yeast,
S. boulardii, is a probiotic that is currently being used by many people.
S. boulardii effectively prevents anti-biotic-related diarrhea, traveler’s diarrhea, and acute diarrhea in adults and is safe and well-tolerated, not causing any side effects.
4 The activity and effects of
S. boulardii are diverse. The yeast produces a 54 kDa protease to degrade C. difficile toxin A and toxin B directly,
5 and a 63 kDA phosphatase to break down the endotoxin of the pathogenic
E. coli.
6 S. boulardii improves the integrity of the tight junctions between the intestinal walls and has metabolic activity that increases the short-chain fatty acids. In addition, it can control the immune response by inhibiting the production of proinflammatory cytokines.
7 Many studies regarding the effects of
S. boulardii are ongoing, and in the future, these characteristics will be used in accordance with the pathophysiology of many gastro-intestinal diseases.
Crohn’s disease (CD) is an immune-related disease characterized by uncontrolled intestinal inflammation. Although various treatments, such as 5-aminosalicylic acid, thiopurine, biologics, and small molecules, are currently being used as the standard therapies, it sometimes leads to disability because of uncontrolled inflammation.
8 Many clinicians believe that probiotics can promote mucosal healing in the intestine against uncontrolled inflammation. Previous studies have shown that in patients with CD,
S. boulardii reduces the intestinal permeability and stabilizes the intestinal wall.
9 Based on this theoretical background, several studies have examined whether the administration of
S. boulardii in CD patients prevents the worsening of the symptoms and helps maintain remission.
10-12
This study examined the changes in the Crohn’s disease activity index (CDAI) after administering S. boulardii to patients with CD in the remission status. In addition, the changes in nutritional status and intestinal inflammation were analyzed using a blood and stool test to assess the safety of administering S. boulardii in CD patients.
Go to :

SUBJECTS AND METHODS
1. Patients and medication
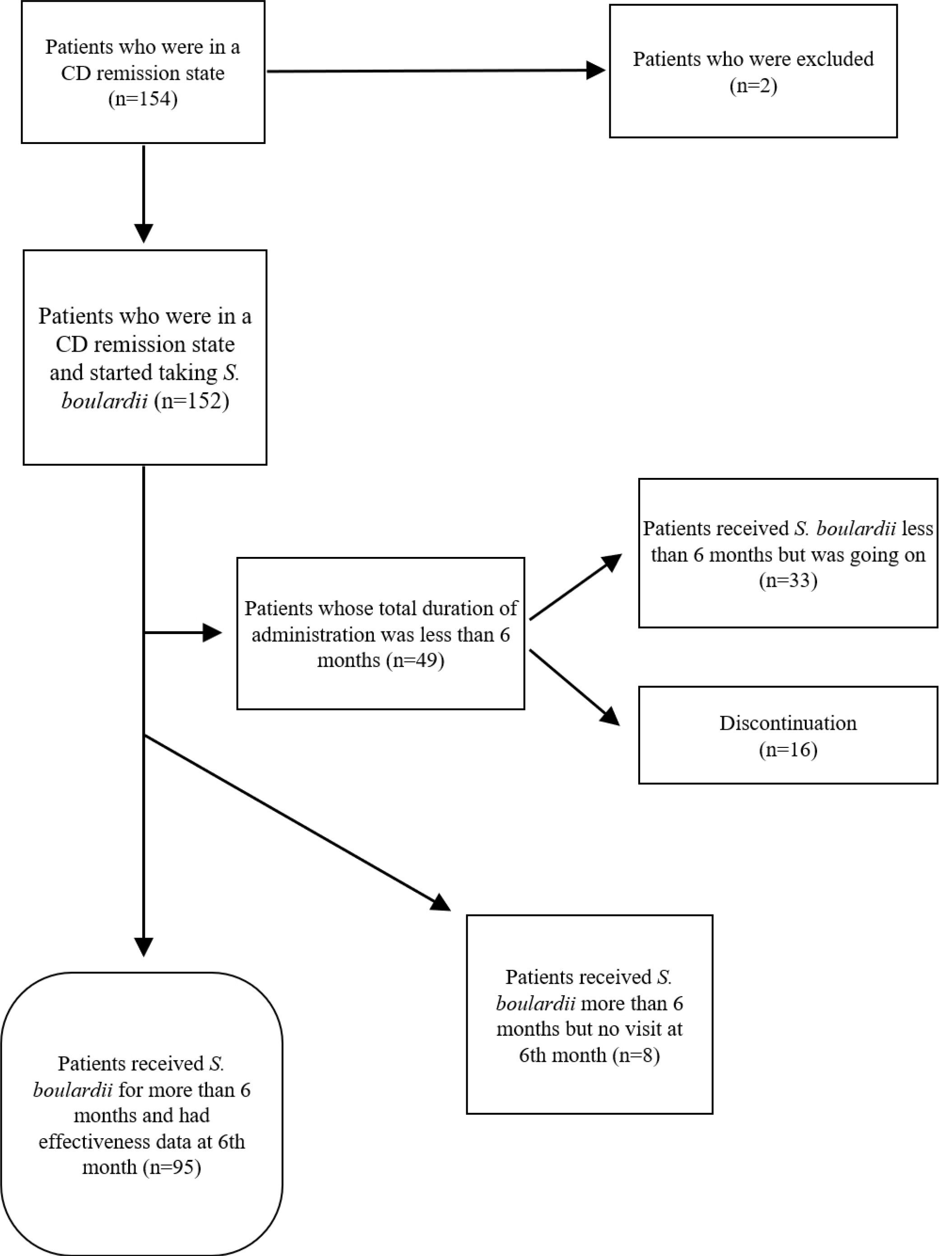
This was an observational retrospective study in a single hospital-based cohort. The study was conducted under the approval of the Institutional Review Board of Kosin University Gospel Hospital in Busan, Korea (IRB No. 2019-05-016). The medical records of all CD patients with a steroid-free remission for at least 3 months and who had been administered S. boulardii (282.5 mg, powder) at the Crohn’s Disease and Ulcerative Colitis Clinic, Kosin University Gospel Hospital, Busan, Korea from December 1, 2013, to November 30, 2018, were reviewed. The CD remission was designated below 150 points of the CDAI. Patients with any malignancy or those who had previously taken any other probiotics were excluded.
2. Measurement of the results
Among the patients enrolled, those who took the drug for more than 6 months were evaluated to determine the effectiveness and safety of S. boulardii. Their CDAI at the baseline and 6 months after administering S. boulardii were compared. The patient’s weight and BMI were identified. Blood and stool samples were compared to evaluate the nutritional status: serum hemoglobin (Hb), ferritin, vitamin B12, folate, iron, albumin, total protein, and total cholesterol; and the intestinal inflammation status (serum [CRP] and fecal calprotectin [FC] level). The number of people who stopped taking the drug within 6 months was checked, and the timing and reason for the discontinuation were investigated.
3. Statistical analysis
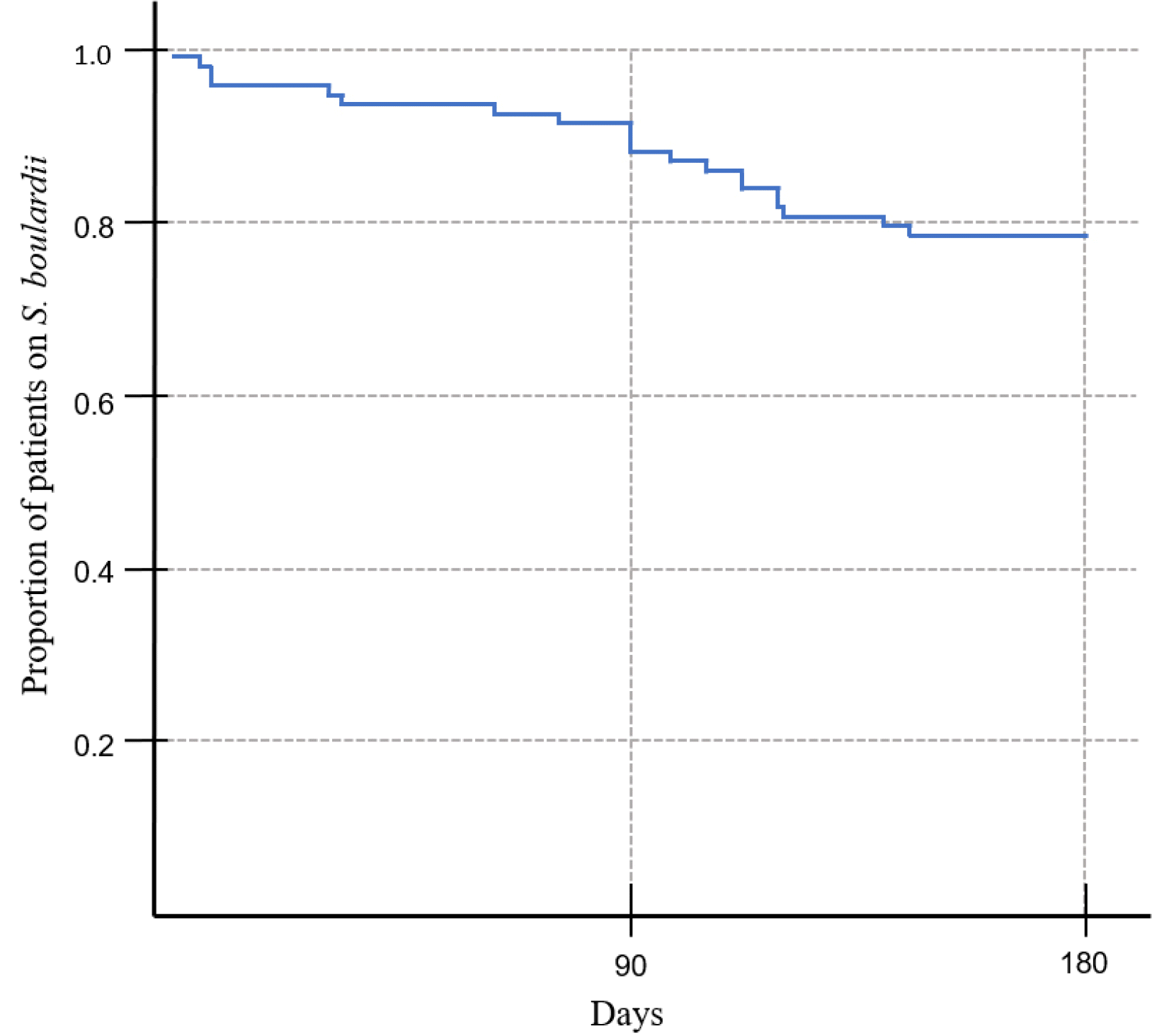
Statistical differences of the CDAI, blood test, and stool test results between the baseline and 6 months after administering the drug were determined. The continuous variables following a normal distribution were compared using a paired-T test. The Wilcoxon signed-rank test was used in the case of a non-normal distribution or categorical variables. The statistical significance was based on the 95% CI (p<0.05). In addition, Kaplan-Meier curves were drawn by examining the number of patients who had discontinued the S. boulardii treatment during the first 6 months. Statistical Package for Social Science (SPSS 21) (IBM Corp, Armonk, NY, USA) was used for the statistical analysis.
Go to :

DISCUSSION
This study was an uncontrolled retrospective study that involved patients with CD at a tertiary university hospital. Among the patients with CD who were in remission for more than 3 months, the medical records of 152 patients administered
S. boulardii were reviewed, and the 95 cases had received the drug for more than 6 months and had all the data for the baseline and at 6 months were examined to compare the differences with the predose period. The CDAI decreased significantly from 40.65 to 32.06 6 months after taking the drug (p<0.01). On the other hand, there was no significant FC from 217.
4 µg/g to 233.1 µg/g. During the period of administration in 152 patients, there was no significant safety issue related to the drug. Sixteen patients stopped within 6 months, and only one of them required hospitalization due to acute exacerbation.
CDAI is a good indicator that reveals the current disease activity of CD, and can be used to judge the patient’s condition in various ways through symptoms, along with the hematocrit and weight. In this study, CDAI decreased by eight points when
S. boulardii was taken for 6 months. The declining results are probably due to an increase in body weight (2.45% increase in ideal body weight) and an increase in the hematocrit (1.56 increase). On the other hand, there was no change in the number of liquid or very soft stools per week, abdominal pain, general well-being, extra-gastrointestinal symptoms, anti-diarrhea drug use, or abdominal mass. There was no significant difference in the number of diarrhea episodes, which contrasts with previous results.
13 This study targeted the patients who were already in a stable remission for more than 3 months. Therefore, it may be difficult to decrease the mean number of diarrhea episodes further from 2.28 of the baseline number.
CDAI decreased by eight points, and
S. boulardii improved the general condition of the CD patients in remission. On the other hand, considering that biologics lowered the CDAI by more than 70 points as maintenance therapy in previous studies,
14 S. boulardii did not lead to intensive changes in clinical practice.
S. boulardii appeared to help patients with CD who frequently develops chronic anemia and underweight.
Patients with CD are in a state of chronic malnutrition. Studies have shown that weight loss occurs in 65-75%, hypo-albuminemia in 25-80%, and anemia in 60-80% of CD patients.
15 There was an increase in BMI, Hb, and total cholesterol after taking
S. boulardii. This may be helpful in patients who have a poor overall nutritional status compared to normal people. Taking
S. boulardii stabilizes the barrier and strengthens the tight junction to decrease the permeability of the intestine, which will help improve the nutritional status. Nutrient factors related to anemia, such as iron, ferritin, and vitamin B12, were not increased. In the previous study, the predictors of the anemia were the serum erythropoietin, transferrin, and soluble transferrin receptor,
16 but these were not included in this study. How the administration of
S. boulardii affects these predictors of anemia correction is unknown. This study was conducted without a control group. Therefore, weight gain and anemia correction might be associated with the maintenance effect of a stable remission status for a long period rather than the only effect of
S. boulardii. On the other hand, it was assumed that
S. boulardii might have contributed in part because many patients were underweight and had a reduced fat mass even in remission.
17
The inflammatory condition did not show any significant changes, as judged by CRP and FC. CRP averages within the period were all within the normal range. Therefore, the judgment on improvement and exacerbation is meaningless. FC increased numerically, but the increase was not significant. FC quantifies the neutrophil activity in the intestinal tract; it indicates the degree of inflammation in the intestine. An increase in FC has significance as a prognostic factor for acute exacerbation of CD. There is no clear consensus yet, when the FC is above 250, the possibility of CD flare-up is generally considered.
18,19 In this study, the average was over 200 µg/g, and the standard deviation was approximately 400 µg/g. This means that intestinal inflammation is still active, even in patients who are in remission, and the variation is large for each patient.
S. boulardii is assumed to regulate pro-inflammatory cytokines and reduce FC and intestinal inflammation. On the other hand, there was no significant decrease in FC. This lack of difference can be attributed to the short observation period of 6 months, where there may be no apparent differences. Considering the characteristics of inflammatory bowel disease, intestinal inflammation is continuously being induced for a long time, eventually leaving the patient with a disability. Long-term use of more than 6 months is required to control the cytokines to change the chronic inflammatory state and eventually change the course of the disease. In addition, probiotics can preoccupy the intestines by forming colonies and pushing the harmful bacteria out of the gut in the case of a pathogenic bacterial invasion. The invasion of pathogenic bacteria causes the flare-up of CD, and the observation period of 6 months is considered short of having an episode of pathogenic bacterial invasion.
Clinicians always pay attention to the safety and stability of probiotics. In general,
S. boulardii is very safe.
4 Fungemia has been reported in patients with severe comorbidities and central lines, but it is rare.
20 In this study, one case was discontinued due to pregnancy while taking the drug. They were very well tolerated in all other cases. In addition, only one patient was hospitalized due to acute exacerbation and required an intravenous systemic steroid treatment. In that case, the effect of
S. boulardii for the CD flare-up appears to be small because it had been administered for 120 days.
The advantage of this study is that
S. boulardii was administered for 6 months to a relatively large number of patients with CD, and the changes in CDAI in real clinical practice were examined. The limitation is that it was a single institutional study and with a retrospective study design. In addition, owing to the nature of CD, the heterogeneity such as the location of the lesion, degree of inflammation, duration of the disease, and the extra-gastrointestinal symptoms is very diverse. Therefore, the effects of administering
S. boulardii cannot be fully explained by the sample size of this study. Generally,
S. boulardii takes approximately three days to settle in the intestine after administration.
21 The evaluation was performed after 6 months of administration, but it was insufficient to evaluate the long-term changes. In the future, controlled, multi-center studies that administer
S. boulardii for a longer time will be necessary.
In conclusion, S. boulardii improves the CDAI in patients with CD in clinical remission and helps stabilize the nutritional status without safety issues. Further randomized controlled studies will be needed through a long-term follow-up.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download