I. Introduction
Cleft lip and/or cleft palate and cleft palate alone are types of orofacial clefts (OC)
1, which are the most common orofacial inborn deformities among live births
2. OC are major congenital malformations in the structure of the oral and maxillofacial region that have lifelong morbidity and an intricate etiology
3.
An alveolar cleft is a well-described inborn malformation with a prevalence of 0.18-2.50 per 1,000 infants
4. Among alveolar clefts, a unilateral cleft is more common. Alveolar clefts occur in 75% of cases of cleft lip
5. Alveolar clefts result from incomplete fusion of the nasal process and oropalatal shelves
6. This abnormality mainly involves the area of the canines and lateral incisors, but can also include the central incisors
7.
This defect is also accompanied by several problems, including tooth eruption within the clefts, oronasal fistulas, and deviation of the alveolar segments. Large defects can cause speech problems
8. Alveolar clefts can differ in severity, but are typically associated with a deficiency of the maxillary bone. Consequently, there is no base for dental growth or preservation of permanent dentition
7.
The guidelines for surgical mending of clefts include: achieving proper closure of the mucosa of the nasal floor to prevent contact between the nose and the oral cavity; repairing this anomaly with bone grafts; and achieving sufficient closure of the oral mucosa on the palatal and labial portions to reach a proper seal over the grafted bone
9.
Bone regeneration is the main research aspect and aim for craniofacial and orthopedic surgeons
10. Bone regeneration was also the motivation for the development of alveolar cleft bone grafting, with the aim of fixing this defect and restoring normal function and aesthetics. Bone regeneration can be grouped into primary (during infancy), secondary (during mixed dentition), and tertiary (after the eruption of the dental arch) bone grafting. The current focus is on secondary bone grafting.
The purpose of bone grafting is to mend a defect or malformation using tissues and biological materials
11. A clinician has multiple options for augmentation that depend on the level of the deficiency: autogenous bone graft (iliac crest, cranium, tibia, mandibular symphysis)
12, interposition bone graft
13, guided bone regeneration, xenografts, and alloplastic materials (bone substitutes)
14.
These sources, in general, have been used to treat patients by placing the bone graft in an alveolar cleft as one step of the intricate sequence of cleft lip and palate repair
15. Skeletal defect grafting is a critical part of the construction of bony flow in the dental arch
16. However, even if the bone grafts are placed in a high volume, there is a possibility that graft resorption, or alveolar notching will occur
17. Bone grafts undergo resorption in three dimensions
18, which mostly appear in autogenous bone grafts due to insufficient cover from the soft tissue
19.
In addition, clinicians encounter bone graft failure due to unforeseen factors, which may lead to the need for additional surgeries. To the authors’ best knowledge, no prior studies have evaluated the factors that can affect alveolar cleft bone grafting, influence its results, or lead to postoperative complications or failure. Therefore, we evaluated several factors to determine if any has an impact on this procedure.
II. Materials and Methods
This retrospective study analyzed the follow-up records, and radiographs of all patients who received alveolar cleft bone grafting at the Department of Oral and Maxillofacial Surgery at Mahidol University, Bangkok, Thailand from January 2014 to December 2018. The study was approved by the Faculty of Dentistry/Faculty of Pharmacy, Mahidol University, and our Institutional Review Board (MU-DT/PY-IRB) with a study approval number of MU-DT/PY-IRB 2019/DT006. Informed consent was not obtained, because it was not possible in this retrospective study that used patient data from years prior. Therefore, no personal data or photographs were included in our data. Therefore, the authors postulated that the patients would not have any objections to the study. The selection criteria are presented in
Table 1.
Two dentists collected the data related in every case. When there was disagreement regarding the x-rays or disease classification, it was resolved through discussion and consensus. Following the completion of data collection, all parameters were listed and rechecked for any missing information.
1. Surgical procedure
Five surgeons performed the secondary or tertiary alveolar cleft bone grafting procedure in all cases. All of them have more than 10 years of experience in the field of oral and maxillofacial surgery. In addition, they all graduated from the same institution and underwent the same training.
All cleft sites received autogenous bone grafts from the iliac crest. The surgical procedures in all cases were performed as follows: the first step was the induction of general anesthesia, which was pursued by performing a conventional sterile draping. A local anesthetic was injected intraorally and in the skin, where the incision will be made to harvest the bone graft.
Surgeons waited for five minutes to allow the effect of the local anesthetic to occur. The surgery was then performed on two surgical sites simultaneously. The type of flap in the cleft site depended on its size and shape. Next, the bone was exposed, and the fibrous tissue was removed. The fistula was closed whenever it existed. In the other surgical site, a skin incision was made, after which the bone of the iliac crest was exposed.
The bone was harvested as a particulate bone and was kept in normal saline until it was used to graft the cleft area. After placing the bone, a flap advancement was performed in all cases to close the flap and cover the bone graft without any tension. No graft fixation was performed, because it was particulate bone. No block grafts were used. Watertight suturing was performed in the end using Vicryl 3-0 (Ethicon, Somerville, NJ, USA) threads intraoally. In contrast, Vicryl 2-0 (Ethicon) was used for thoracolumbar fascia and subcutaneous tissue, and nylon 6-0 (Ethicon) for the skin was used in the area of bone graft harvesting.
2. Variables
The outcome variables for this surgery were divided into three groups. Group A included cases in which there were no complications for up to one year postoperatively. The complications in group A were within types 1 or 2 according to the Bergland scale
20 at one year of follow up. Group B included cases in which there were complications during the first year of follow up, or those reaching Bergland type 3.
The complications were listed as wound dehiscence, infection, inflammation, bone exposure, and oronasal fistula formation. Group C was mainly the failure group that reached Bergland type 4 with the need for another surgery to graft the area again. The groups are explained in
Table 2.
1) Host factors
The host factors included sex (male, female), and the age of the patient when undergoing surgery. The patients were divided into group one (less than 12 years old), and group two (more than 12 years old).
2) Pathology factors
The pathology factors included the type and size of the cleft. These factors were divided into groups in order to perform the statistical analysis and determine their significance by comparing the change within each factor that led to an effect or not. The type of cleft was grouped into bilateral or unilateral. The size of the cleft was divided into <10 mm as group one, and >10 mm as group two. When placing cases with a bilateral cleft in those two size groups, each side of the cleft was considered a single side.
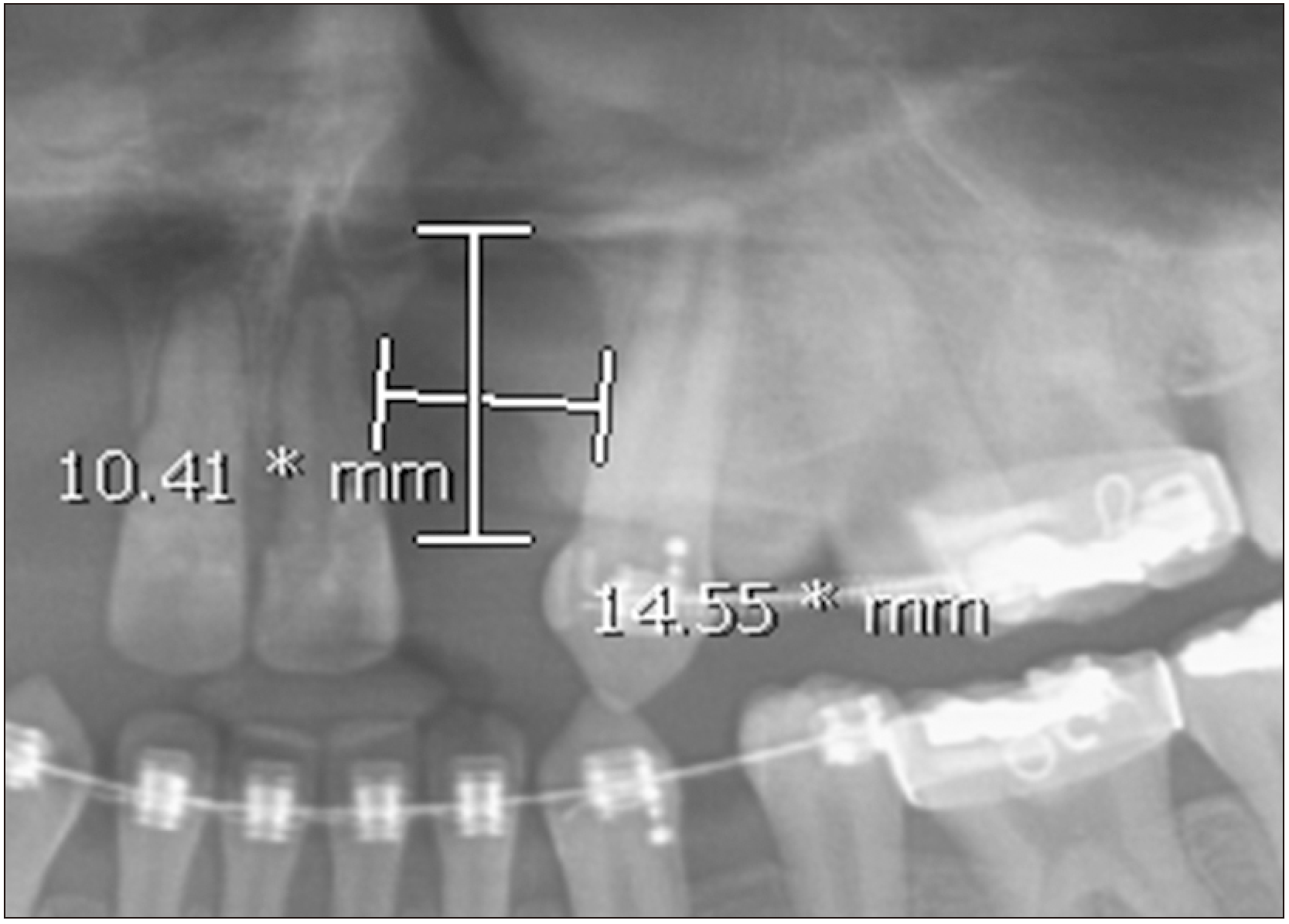
Given the lack of cone-beam computed tomography (CBCT) scans in most cases, using CBCT in some cases and panoramic radiographs in others would have created a conflict in relation to measuring the cleft size in all cases. Therefore, only the panoramic radiographs were used to perform this step. The widest areas of the cleft horizontally and vertically were chosen to determine the size in those two dimensions.(
Fig. 1)
3) Treatment factor
The treatment factor was the type of flap used in surgery. The type one flap was the trapezoidal sliding flap with a palatal flap. The other kinds of flaps were all specified as type two, given the low number of other types.
3. Statistical analysis
Univariable and multivariable multinomial logistic regression were performed to evaluate the significance of all factors included in this study. The P-value was set at 0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows (ver. 24; IBM, Armonk, NY, USA).
III. Results
Sixty-seven cases in total were included in this study. Twenty-six patients were males and 41 were females. However, there was missing data related to certain factors in a number of cases. Therefore, every factor had a different sample size. The general characteristics of each group are explained in
Table 3.
Thirty-one patients were under 12 years old, and 34 were over 12 years old. The youngest patient among them was 8 years old, while the oldest was 36 years old. The trapezoidal sliding flap with a palatal flap was performed in 54 surgeries, while other kinds of flaps were performed in the other 12 cases.
Fifty-two patients had a unilateral cleft, while 15 had a bilateral cleft. Sixteen patients had a cleft that was <10 mm in size, while 39 cases had a cleft of >10 mm in one of the dimensions.
There were thirty-two cases in group A, thirteen patients were male and nineteen patients were female. Thirteen of these patients were under 12 years old, while 18 were over 12 years old. One patient had missing age data. Twenty-seven surgeries were performed using the trapezoidal sliding flap with the palatal flap, while four surgeries were performed with other types of flaps. One of these cases also had missing data regarding the flap used. Three cases involved bilateral clefts, while 29 cases were unilateral. Thirteen clefts were <10 mm in size (considering the two dimensions). In contrast, 13 clefts were >10 mm in size, with six cases with missing size information.
Twenty-six cases were listed in group B. These cases included 9 males and 17 females. Of these cases, fourteen patients were over 12 years old and 11 were under 12 years old, with one case missing age data. Nineteen surgeries were performed using the trapezoidal sliding flap with a palatal flap and 7 were performed using other kinds of flaps. Nine cases were bilateral and 17 were unilateral clefts. Three cases were <10 mm in size, while 18 cases were >10 mm in size.
Nine cases were in group C, including 4 males and 5 females. Of these, four patients were under 12 years of age and 5 patients were over 12. Eight cases were treated using the trapezoidal sliding flap and one with another kind of the flap. Three cases were bilateral and six were unilateral. Eight clefts were >10 mm in size, while none were <10 mm in size.
1. Factors that have significance and a possible effect
Univariate ordinal logistic regression analysis showed size and type of the cleft were significant between groups, including unilateral or bilateral types. Nine of the 15 cases of bilateral clefts were in group B, which means that those cases had complications during the 1 year follow up period, or reached type 3 (on the Bergland scale) by the end of the year.
In addition, three cases were in group C, meaning that those cases reached type 4 on the Bergland scale and required additional bone grafting surgery. Among the 39 cases that were >10 mm in size, eighteen were in group B, and 8 were in group C.
Multinomial logistic regression analysis showed there was a meaningful correlation between bilateral cleft and having postoperative complications or Bergland type 3 status. Furthermore, clefts >10 mm in size were associated with group B (complications or were Bergland type 3). Due to the small sample size, an analysis could not be performed to evaluate any relationship with group C.
The age, sex, and type of flap used in surgery were not associated with groups B or C. In other words, there was no relationship between those factors and having postoperative complications, or reaching type 3 or 4 on the Bergland scale. All of the results are shown in
Table 4.
IV. Discussion
The alveolar cleft takes place in response to abnormal development within the stages of frontonasal prominence growth, proximity, and fusion
17. Alveolar cleft repair is mainly performed by grafting with autogenous bone, as well as several tissue-engineered materials
21. The main source of autogenous bone is the iliac crest, because it offers the possibility of fairly easily collecting a relatively high volume of bone
22. In this study, all of the clefts were grafted using iliac crest bone. Therefore, it was not possible to put this factor (source of bone graft) into the analysis.
Twenty-six cases were in group B, while nine cases were in group C. However, it is important to recognize that complications can occur at any time, and this does not reflect failure. Therefore, surgeons must be prepared for complications, deal with them promptly, and be familiar with potential factors that can contribute to complications.
Similarly, failure should not be thought of as an unavoidable event. However, one must still have an idea of when failure is more likely to occur so that he/she can manage it. This recognition will also allow for better communication between the parents and their child’s surgeon, as well as between the patients and their surgeon in case of adult patients. This study was performed with the goal of identifying some of the factors related to complications and failure.
Several studies have mentioned that the golden period for performing alveolar cleft bone grafting is during the mixed dentition stage
23>-25. Performing bone grafting at this stage helps to establish bone continuity, stabilize the maxillary arch form, support the nasal base by augmenting the piriform rim, close the oronasal fistula, and eventually, build the path for permanent teeth eruption in the cleft space
26. Unexpectedly
27, we found that some of the main aspects can still be achieved even if a patient has crossed the mixed dentition stage.
The patients in group A who were >12 years old attained bone continuity and healing without any graft rejection or complications. Therefore, these patients achieved stabilization of the maxillary arch. In addition, there was no fistula formation up to one year postoperatively.
This study shows that undergoing alveolar cleft bone grafting at a later stage can still produce good results and is considered a success. In particular, reports regarding the eruption of the canines in the graft site were inconsistent in the literature
28,29. This result is similar to those of Murthy and Lehman
30, who found that there was no statistical correlation between age and complications. Nonetheless, it was not in agreement with the result of this study with respect to the type of cleft, as it was not correlated with complications.
In this study, having a bilateral cleft was associated with an odds ratio of more than five of being included in group B, and an odds ratio of approximately 5 of being in group C. Therefore, patients with bilateral clefts should be informed about their specific risk of postoperative complications, losing a noticeable amount of bone from a graft, or even the need for a reoperation. This chance is approximately 5 times higher than that of other patients with unilateral clefts. One possible reason for this disagreement in the two studies is that x-rays were taken approximately six months after grafting in the other study. However, in this study, the follow up lasted for 1 year, and x-rays were provided at that time.
The type of flap used in surgery, and the patient’s sex were not significantly associated with postoperative complications or reaching type 3 or 4 on the Bergland scale. This was in consensus with another study, which analyzed the risk factors related to graft failure after cyst enucleation
31.
We also found that sex was not correlated with graft failure. Although mentioning a different recipient site, Zuo et al.
32 used the iliac crest bone to graft the femoral head-neck junction for the treatment of osteonecrosis of the femoral head. When investigating the failure, their results also showed no effect of age on the clinical outcome of the surgery.
The flap design is mainly dependent on the size, type, area, and shape of the cleft. Every flap has its advantages and disadvantages. The choice of flap is also related to the surgeon’s experience and preference. The important points to consider are creating a mucoperiosteal flap that can be adequately elevated, and sealed without producing any tension on the tissues. In addition, it is important to achieve a sufficient cover over the grafted bone particles to ensure that the graft will not be exposed
17.
The types of flap used in this study included the trapezoidal sliding flap, palatal flap, finger flap, or sulcular incision along the cleft with buccal flap advancement when the size was small. The trapezoidal sliding flap with palatal flap was used in most cases. Therefore, all of the cases with that kind of flap were included in one group, while the remainder of cases was included in another group. There was no significant difference in these two groups, which indicated that there is no association between the flap design and the postoperative outcome of the surgery.
A size of >10 mm was associated with the presence of complications or reaching type 3 according to Bergland scale with an odds ratio of six. This finding is also a notable factor that a patient must be informed about.
The chance of reaching type 3 is approximately six times higher in a patient with a cleft >10 mm than it is in a patient with a cleft <10 mm in size. Due to the small sample, statistical analysis could not be performed to evaluate the association between the size of the cleft and graft failure. However, the authors suspect that there is a relationship between both with a high odds ratio. In particular, prior reports have suggested that there is a correlation between substantial defects following cyst enucleation and graft failure
33,34. The analysis of Lim et al.
31 is similar to the one in this study.
All of the involved surgeons were trained at the same institution, and taught by the same teachers. Therefore, the steps and standards used in each surgery were almost identical.
One limitation of this study is its failure to include more factors related to patient outcomes. For instance, other potential factors that may be related to surgical outcomes include: smoking, systemic disease, alcohol consumption, the surgical time, preoperative infection, oral hygiene postoperatively, previous infection, or even the surgeon who performed the bone grafting.
Those factors may have a great impact on the case prognosis. It would have been beneficial to add those factors; however, it was not possible to include all of these factors given the small sample size. Furthermore, as previously mentioned, the analysis could not be performed with regard to one of the included factors (cleft size of >10 mm) due to this limitation.
This concern was raised at the beginning of our work. Regardless, we sought to determine the important factors to consider and be aware of when performing alveolar cleft bone grafting. We also believe that these factors are important to discuss with patients regarding the potential complications or failure when those factors present. In addition, this study serves as the base of further studies to confirm what has been observed. Further studies with a larger sample size would give a clearer vision about what was stated in this research, in an attempt to confirm some of the important factors that should be considered when performing such a procedure.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download