Introduction
Materials and Methods
Subjects
Statistical Analysis
Results
Changes of Nodule Size According to the Duration of Follow-Up
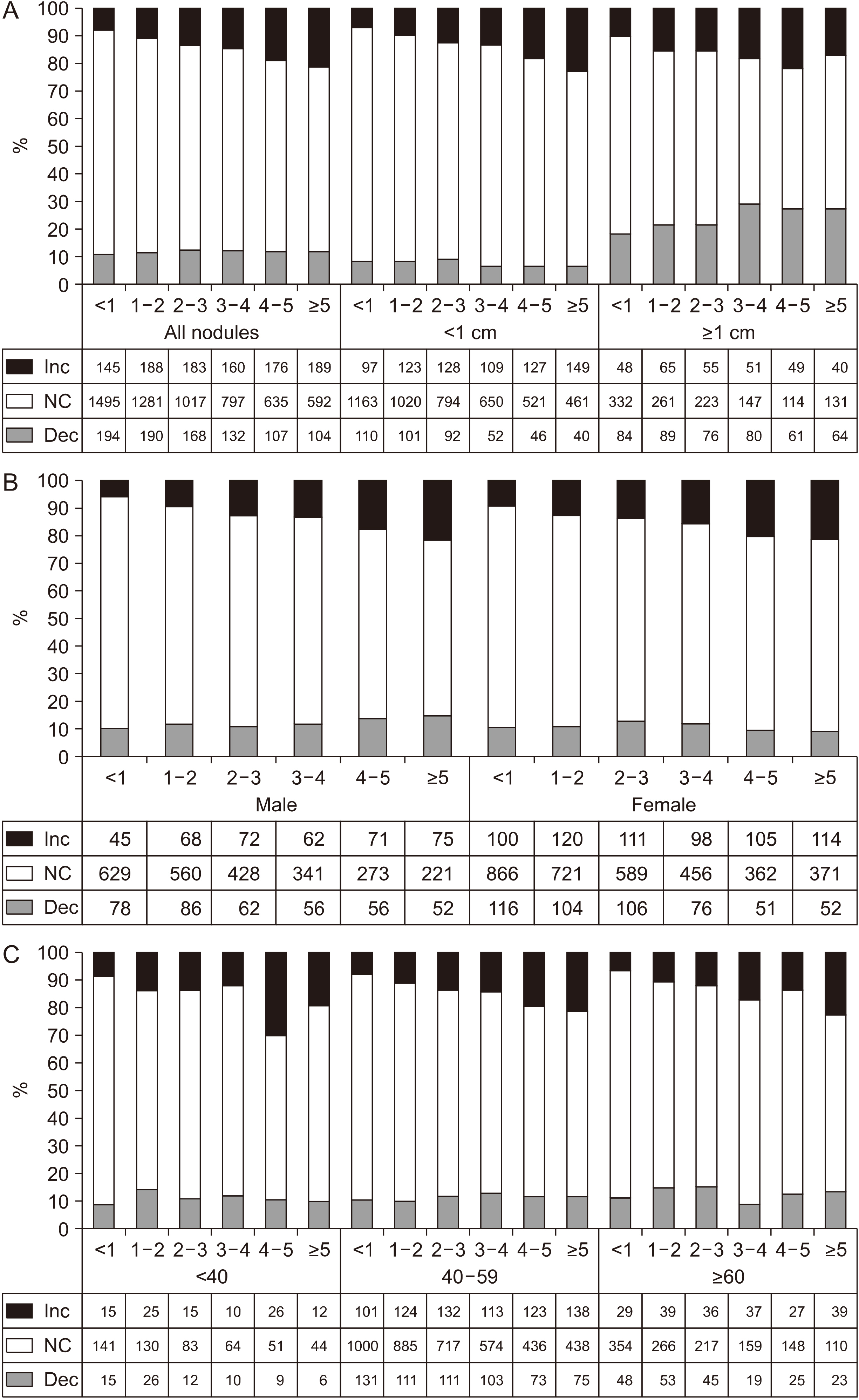 | Fig. 1The rate of change in the size of the thyroid nodules according to the follow-up period (years), the numbers (<1, 1-2, 2-3, 3-4, 4-5, >5) on the horizontal axis indicate the duration of follow-up of the nodule. (A). All nodules and when divided by size (1 cm). (B) When divided by gender. (C) When divided by age categories (<40, 40-59, ≥60 years). Dec: decrease, Inc: increase, NC: no change |
Comparison of Baseline Demographic or Clinical Characteristics of Subjects According to the Change of Noduar Size
Table 1
| Size of thyroid nodule | Total | No change | Decreased† | Increased† |
|---|---|---|---|---|
| N (%) | 7753 | 5817 (75.0) | 895 (11.5) | 1041 (13.5) |
| Sex | ||||
| Male (%) | 3235 (41.7) | 2452 (42.2) | 390 (43.6) | 393 (37.8)§ |
| Female (%) | 4518 (58.3) | 3365 (57.8) | 505 (56.4) | 648 (62.2)§ |
| Age (years), mean±SD | 52.1±9.4 | 52.1±9.4 | 52.1±9.5 | 51.7±9.5 |
| <40 | 694 (9.0) | 513 (8.8) | 78 (8.7) | 103 (9.9) |
| 40-59 | 5385 (69.5) | 4050 (69.6) | 604 (67.5) | 731 (70.2) |
| ≥60 | 1674 (21.5) | 1254 (21.6) | 213 (23.8) | 207 (19.9) |
| Numbers of thyroid ultrasound, mean±SD | 3.1±1.4 | 3.0±1.4 | 2.9±1.3 | 3.5±1.7§ |
| Duration of follow up (months), mean±SD (median, ranges) |
32.4±20.7 (27.0, 3.0-91.3) |
30.8±20.3 (25.7, 3.0-91.3) |
36.1±21.1‡ (35.2, 3.0-87.4) | 39.1±21.3§ (37.1, 3.0-91.3) |
| Size at baseline (mm), mean±SD | 8.2±5.7 | 7.5±4.9 | 11.5±7.3‡ | 9.1±7.1§ |
| <5.0 | 2165 (27.9) | 1750 (30.1/80.8) | 125 (14.0/5.8)‡ | 290 (27.9/13.4)§ |
| 5.0-9.9 | 3618 (46.7) | 2859 (49.1/79.0) | 316 (35.3/8.7)‡ | 443 (42.6/12.2)§ |
| 10.0-19.9 | 1595 (20.6) | 1029 (17.7/64.5) | 346 (38.7/21.7)‡ | 220 (21.1/13.8)§ |
| ≥20.0 | 375 (4.8) | 179 (3.1/47.7) | 108 (12.1/28.8)‡ | 88 (8.5/23.5)§ |
| Diffuse parenchymal abnormality (%)* | 8.2 | 6.7 | 15.3‡ | 10.5§ |
| TSH (mU/L), mean±SD | 2.0±3.1 | 2.0±3.4 | 2.1±2.6 | 1.9±1.8§ |
| <0.4 | 193 (2.6) | 118 (2.1) | 38 (4.5)‡ | 37 (3.7)§ |
| 0.4-4.1 | 6831 (91.8) | 5183 (92.5) | 752 (89.1)‡ | 896 (89.7)§ |
| >4.1 | 420 (5.6) | 300 (5.4) | 54 (6.4)‡ | 65 (6.6)§ |
| Free T4 (ng/dL), mean±SD | 1.24±0.30 | 1.23±0.28 | 1.26±0.43 | 1.23±0.24 |
| Smoking (ex-smoker or current smoker) (%) | 38.1 | 38.3 | 37.8 | 36.9 |
| Systolic blood pressure (mmHg), mean±SD | 117.9±16.2 | 117.7±16.1 | 118.4±15.6 | 118.4±16.8 |
| Diastolic blood pressure (mmHg), mean±SD | 75.8±11.9 | 75.7±11.9 | 76.4±11.6 | 76.2±12.2 |
| Body mass index (kg/m2), mean±SD | 23.4±2.9 | 23.4±2.9 | 23.6±2.9 | 23.4±2.9 |
| Waist circumference (cm), mean±SD | 84.5±8.1 | 84.5±8.1 | 84.9±8.1 | 84.3±8.1 |
| Body fat (%) | 26.5±5.7 | 26.4±5.7 | 26.7±5.7 | 27.0±5.6 |
| Fasting glucose (mg/dL) | 98.1±17.9 | 97.9±17.9 | 98.7±17.3‡ | 98.7±18.0 |
| HbA1c | 5.74±0.60 | 5.75±0.61 | 5.75±0.54 | 5.72±0.61 |
| <6.5% | 6791 (92.9) | 5128 (93.1) | 765 (93.1) | 898 (91.7) |
| ≥6.5% | 520 (7.1) | 382 (6.9) | 57 (6.9) | 81 (8.3) |
| Total cholesterol (mg/dL) | 198.2±34.1 | 197.8±34.0 | 200.8±33.9‡ | 198.7±35.2 |
Table 2
| Total | No change | Decreased† | Increased† | |
|---|---|---|---|---|
| N | 1970 | 1208 (61.3) | 454 (23.0) | 308 (15.6) |
| Sex | ||||
| Male (%) | 784 (39.8) | 465 (38.5) | 203 (44.7)‡ | 116 (37.7) |
| Female (%) | 1186 (60.2) | 743 (61.5) | 251 (55.3) | 192 (62.3) |
| Age (years old) | 53.2±9.7 | 53.4±9.7 | 53.7±9.6 | 52.0±10.1§ |
| <40 | 145 (7.4) | 83 (6.9) | 30 (6.6) | 32 (10.4) |
| 40-59 | 1310 (66.5) | 809 (67.0) | 293 (64.5) | 208 (67.5) |
| ≥60 | 515 (26.1) | 316 (26.2) | 131 (28.9) | 68 (22.1) |
| Numbers of thyroid sonography | 3.0±1.4 | 2.9±1.4 | 3.2±1.5‡ | 3.3±1.7§ |
| Duration of follow up (months)mean±SD (median, ranges) |
32.5±21.1 (27.8, 3.0-90.7) |
30.2±20.8 (25.2, 3.0-90.7) |
35.6±21.2‡ (35.1, 3.0-87.4) | 36.1±20.5§ (35.0, 3.0-85.9) |
| Initial nodule size (mm) | 15.6±6.5 | 14.9±5.9 | 16.5±7.0‡ | 17.0±7.7§ |
| 10.0-19.9 | 1595 (81.0) | 1029 (64.5) | 346 (21.7)‡ | 220 (13.8)§ |
| ≥20.0 | 375 (19.0) | 179 (47.7) | 108 (28.8) | 88 (23.5) |
| Diffuse parenchymal abnormality (%) | 8.6 | 7.5 | 10.8 | 9.6 |
| TSH (mU/L) | 1.93±2.76 | 1.91±2.87 | 2.10±3.16 | 1.78±1.27 |
| <0.4 | 65 (3.6) | 31 (2.8/47.7) | 16 (3.9/24.6) | 18 (6.4/27.7)§ |
| 0.4-4.1 | 1654 (91.3) | 1030 (92.5/62.3) | 375 (90.4/22.7) | 249 (88.0/15.0) |
| >4.1 | 93 (5.1) | 53 (4.8/57.0) | 24 (5.8/25.8) | 16 (5.7/17.2) |
| Free T4 (ng/dL) | 1.24±0.33 | 1.23±0.33 | 1.25±0.38 | 1.25±0.25 |
| Smoking (ex-smoker or current smoker) (%) | 36.9 | 36.5 | 38.0 | 36.4 |
| Systolic blood pressure (mmHg) | 118.9±16.4 | 118.5±16.6 | 119.8±16.0 | 119.2±16.4 |
| Diastolic blood pressure (mmHg) | 76.0±11.6 | 75.6±11.8 | 77.2±11.3‡ | 75.7±11.3 |
| Body mass index (kg/m2) | 23.6±2.9 | 23.6±2.9 | 23.7±3.0 | 23.7±2.8 |
| Waist circumference (cm) | 85.0±8.2 | 85.0±8.2 | 85.2±8.3 | 84.7±8.2 |
| Body fat (%) | 27.1±5.8 | 27.0±5.9 | 27.0±5.7 | 27.7±5.5 |
| Fasting glucose (mg/dL) | 99.4±19.2 | 98.6±20.7 | 98.6±15.5 | 99.8±17.9 |
| HbA1c | 5.79±0.64 | 5.81±0.69 | 5.73±0.50 | 5.76±0.65 |
| <6.5% | 1627 (91.4) | 993 (90.5) | 379 (93.6) | 255 (91.4) |
| ≥6.5% | 154 (8.6) | 104 (9.5) | 26 (6.4) | 24 (8.6) |
| Total cholesterol (mg/dL) | 198.4±34.9 | 197.4±34.3 | 200.4±33.9 | 199.3±38.1 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download