INTRODUCTION
Parry–Romberg syndrome (PRS) is an acquired degenerative condition characterized by a slow and progressive but self-limited atrophy of the unilateral facial tissues, including the skin, muscles, bones, and cartilage.
1-4 The prevalence of PRS is 1 in 250,000 in the general population, with slight female predominance.
5 It usually becomes apparent during the first decade or early second decade of life.
5 Numerous causes have been suggested including trauma, infection, genetic factor, peripheral and trigeminal neuritis, lymphocytic neurovasculitis, localized scleroderma, endocrine disturbance, autoimmunity, hyper- or hypo-activity of the sympathetic nervous system, and disturbance in fat metabolism of the cerebellum.
2,3,6,7
The primary manifestations of PRS include enophthalmos, dark pigmentation on the cheek or frontal area, a sharp demarcation line (coup de sabre), a localized form of scleroderma, sunken appearance and asymmetry of the face, neurological symptom, and missing teeth and morphological abnormalities in the dentition; these result in aesthetic, functional and psychological problems.
2-4,8-10 Therefore, a multidisciplinary treatment protocol involving dermatologists, surgeons, dentists, and psychologists should be applied to correct the soft tissue deformities and reconstruct the skeletal framework.
11,12
There have been numerous case reports or reviews of a single PRS case or cases exhibiting diverse dental phenotypes (congenitally missing teeth, delayed eruption/impaction, abnormal crown and root morphology, or malocclusion)
8,10,13-19 and treated by orthodontic approaches.
11,20,21 However, these studies did not show the exact entity of the dental phenotypes, treatment modality (Tx-Mod) and its timing, and course of long-term treatment. The reasons are as follows: First, those studies included only one or two cases of PRS. Second, the degree of severity of the PRS phenotype is highly variable. Third, because atrophy of the involved tissues is slow and progressive but self-limited, it is necessary to investigate the type of Tx-Mod and its timing using long-term follow-up data. Therefore, the purpose of this retrospective chart review study was to investigate the dental phenotypes and Tx-Mods in Korean patients with PRS using longitudinal data and a novel PRS severity index.
Go to :

MATERIALS AND METHODS
This retrospective study was reviewed and approved by the Institutional Review Board of the Seoul National University Dental Hospital (SNUDH) (CRI19014). The written informed consents were obtained from patients. The samples consisted of 10 unrelated Korean patients with unilateral PRS (four males and six females; mean age at the first consultation, 11.4 ± 5.7 years; involvement side, five right and five left sides;
Table 1). The inclusion criteria was as follows: (1) patients who were diagnosed as PRS; (2) patients who were treated and/or followed-up at the Department of Orthodontics, SNUDH between 1998 and 2019; and (3) patients whose charts, radiographs, and clinical photographs were available.
Table 1
Numbers of the atrophy-involved area, asymmetry-involved item for calculating the Parry–Romberg syndrome (PRS) severity index
|
Patient |
Sex/
affected
side |
Age
at the
first
consultation
(T0) |
Atrophy-involved area
(each × 10; total, 120) |
|
Asymmetry-involved item
(each × 30; total, 120) |
Sum of
score
and PRS
severity
index |
|
|
|
Soft tissue atrophy |
|
Hard tissue atrophy |
Number
and
score of the atrophy-involved
area |
Facial
soft
tissue volume
difference |
Occlusal plane cant |
Chin point deviation |
Deviation
of oral
commissure |
Number
and
score of the asymmetry-
involved
item |
|
|
|
Forehead |
Periorbital tissue |
Mid
1/3
(nose, upper
lip,
cheek) |
Lower
1/3
(lower
lip,
chin, angle) |
Cranial bone |
Orbitzygoma |
Maxilla |
Mandible |
|
|
Condyle |
Ramus |
Coronoid process |
Body |
Angle |
|
#1 |
F/Right |
12Y 5M |
× |
× |
○ |
○ |
|
× |
× |
× |
× |
○ |
× |
× |
○ |
|
4 (40) |
|
○ |
× |
○ |
× |
2 (60) |
100
(mild) |
|
#2 |
M/Right |
21Y 11M |
○ |
○ |
○ |
○ |
|
× |
○ |
○ |
× |
× |
× |
× |
× |
|
6 (60) |
|
○ |
○ |
○ |
○ |
4 (120) |
180
(severe) |
|
#3 |
M/Left |
4Y 4M |
× |
× |
○ |
○ |
|
× |
× |
○ |
× |
○ |
× |
× |
× |
|
4 (40) |
|
○ |
○ |
○ |
○ |
4 (120) |
160
(moderate) |
|
#4 |
F/Right |
7Y 6M |
× |
○ |
○ |
× |
|
× |
× |
× |
× |
× |
× |
× |
× |
|
2 (20) |
|
○ |
○ |
○ |
× |
3 (90) |
110
(mild) |
|
#5 |
M/Right |
11Y 2M |
× |
× |
○ |
○ |
|
× |
× |
○ |
○ |
× |
× |
× |
× |
|
4 (40) |
|
○ |
○ |
○ |
○ |
4 (120) |
160
(moderate) |
|
#6 |
F/Right |
3Y 6M |
× |
○ |
○ |
○ |
|
× |
○ |
○ |
○ |
○ |
× |
○ |
× |
|
8 (80) |
|
○ |
○ |
○ |
○ |
4 (120) |
200
(severe) |
|
#7 |
F/Left |
10Y 6M |
○ |
○ |
○ |
○ |
|
× |
○ |
○ |
× |
○ |
× |
○ |
○ |
|
9 (90) |
|
○ |
○ |
○ |
○ |
4 (120) |
210
(severe) |
|
#8 |
M/Left |
10Y 2M |
× |
× |
○ |
× |
|
× |
× |
○ |
× |
× |
× |
× |
× |
|
2 (20) |
|
○ |
○ |
○ |
○ |
4 (120) |
140
(mild) |
|
#9 |
F/Left |
14Y 9M |
× |
○ |
○ |
○ |
|
× |
○ |
○ |
○ |
○ |
× |
○ |
× |
|
8 (80) |
|
○ |
○ |
○ |
○ |
4 (120) |
200
(severe) |
|
#10 |
F/Left |
17Y 3M |
○ |
× |
○ |
○ |
|
× |
× |
○ |
○ |
○ |
× |
○ |
○ |
|
8 (80) |
|
○ |
○ |
○ |
○ |
4 (120) |
200
(severe) |
|
Sum |
|
|
n = 3 (30%) |
n = 5 (50%) |
n = 10 (100%) |
n = 8 (80%) |
|
n = 0 (0%) |
n = 4
(40%) |
n = 8 (80%) |
n = 4
(40%) |
n = 6 (60%) |
n = 0
(0%) |
n = 4 (40%) |
n = 3 (30%) |
|
|
|
n = 10 (100%) |
n = 9 (90%) |
n = 10 (100%) |
n = 8
(80%) |
|
|

In patients with PRS, soft tissue atrophy can be defined by the skin texture, color, and volume (
Figure 1), while hard tissue atrophy can be defined by the size and shape of the skeleton (
Figure 2). However, since it is difficult to objectively rate the degree of atrophy, we used the following as simple and objective criteria: soft tissue atrophy in the forehead, peri-orbital tissue, middle and lower thirds of the face (
Figure 1) and hard tissue atrophy in the cranial bone, orbit-zygoma, maxilla, and mandible (
Figure 2). Facial asymmetry can be evaluated on the basis of four parameters: difference in the facial soft tissue volume between the affected and unaffected sides, deviation of the oral commissure, occlusal plane cant, and chin point deviation (
Figures 1 and
2). Although these parameters can be evaluated by clinical photographs and cephalometric analysis, the degree of asymmetry can change with the progression of atrophy and growth of the unaffected side. Therefore, the numbers of atrophy-involved area and asymmetry-involved item may be more important than the degree of atrophy and asymmetry in clinical assessment for the classification of PRS.
 | Figure 1Cephalometric and panoramic radiographs acquired from patients with Parry–Romberg syndrome (PRS) stratified by the PRS severity index. A, A patient with mild PRS (patient #8, left side involvement). B, A patient with moderate PRS (patient #5, right side involvement). C, A patient with severe PRS (patient #9, left side involvement). 
|
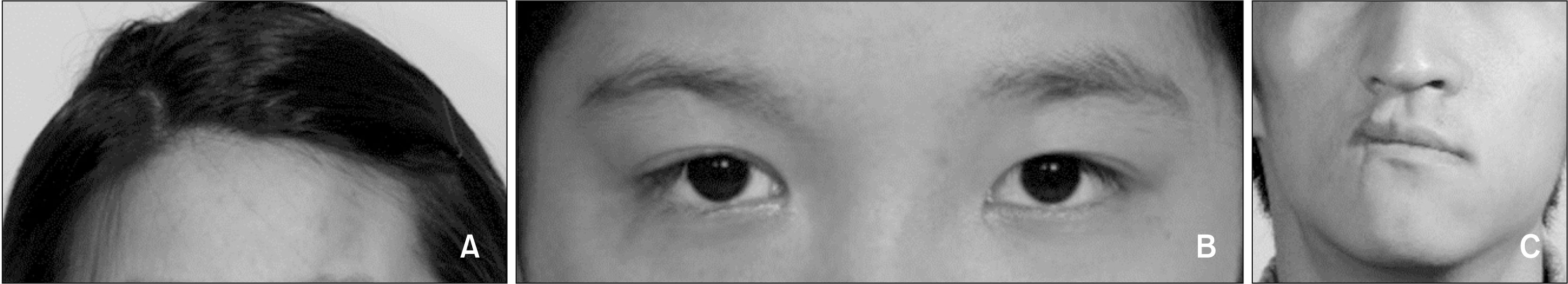
 | Figure 2Facial photographs showing soft tissue atrophy and facial asymmetry in patients with Parry–Romberg syndrome. A, Soft tissue atrophy in the forehead (patient #7, left side involvement). B, Soft tissue atrophy in the peri-orbital tissue (patient #6, right side involvement). C, Soft tissue atrophy in the middle and lower thirds of the face, with a difference in the facial soft tissue volume between the affected and unaffected sides, deviation of the oral commissure, and chin point deviation (patient #2, right side involvement). 
|
Using longitudinal data from chart records, radiographs, and clinical photographs, we counted the number of atrophy-involved area (soft tissue atrophy in the forehead, peri-orbital tissue, middle and lower thirds of the face; and hard tissue atrophy in the cranial bone, orbit-zygoma, maxilla, and condyle, ramus, coronoid process, body, gonial angle of the mandible;
Figures 1 and
2) and the asymmetry-involved item (difference in the facial soft tissue volume between the affected and unaffected sides, occlusal plane cant, deviation of the oral commissure, and chin point deviation;
Figures 1 and
2) to determine the PRS severity index (
Table 1). The mandible was divided into several regions because the different structures (condyle, ramus, coronoid process, body, and gonial angle) can significantly influence the degree of facial asymmetry in PRS patients.
Subsequently, the PRS severity index was calculated according to the number of atrophy area (each involved area was counted as 10, total was 120) and the number of asymmetry-involved item (each asymmetry was counted as 30; total was 120) and used to classify PRS into three types: mild (total score, < 140), moderate (total score, between 140 and 170), and severe (total score, ≥ 180).
We also investigated dental phenotypes including congenitally missing teeth, short root, microdontia, dilacerated root, and delayed eruption/impacted tooth, along with Tx-Mod types for each patient. Six Tx-Mods were defined according to the degree of invasiveness and complexity: Tx-Mod-1, growth observation due to mild atrophy and facial asymmetry; Tx-Mod-2, unilateral functional appliance treatment; Tx-Mod-3, fixed orthodontic treatment; Tx-Mod-4, growth observation due to a definite need of surgical intervention; Tx-Mod-5, autogenous or alloplastic grafting using fat, microvascular tissue, bone, silicone, or porous polyethylene implants (MEDPOR; Stryker, Freiburg, Germany); and Tx-Mod-6, orthognathic surgery combined with fixed orthodontic treatment.
Go to :

RESULTS
Distribution of the atrophy area (Table 1)
With regard to soft tissue atrophy, involvement of the forehead and peri-orbital tissue was relatively less frequent than involvement of the middle and lower thirds of the face (30% and 50% vs. 100% and 80%). In terms of hard tissue atrophy, none of the patient showed involvement of the cranial bone. However, the orbit-zygoma and maxilla were involved at 40% and 80% of the patients, respectively. In the mandible, the ramus, condyle, mandibular body, and gonial angle were involved in 60%, 40%, 40% and 30% of the patients, respectively.
Distribution of the asymmetry-involved item (Table 1)
Difference in the facial soft tissue volume and chin point deviation were the most prevalent (100% each), followed by occlusal plane cant (90%) and deviation of oral commissure (80%).
Distribution of PRS severities according to the PRS severity index (Table 1)
Three patients had a mild PRS (patients #1, 4, and 8;
Figure 2A); two had moderate PRS (patients #3 and 5;
Figure 2B); and five had severe PRS (patients #2, 6, 7, 9, and 10;
Figure 2C).
Occurrence of congenitally missing tooth (Table 2 and Figure 3)
A total of 29 congenital missing teeth was observed in six of the 10 patients (60%; mean, 4.83 missing teeth per patient). All six patients (100%) exhibited congenitally missing teeth in the maxillary arch (n = 19), while three patients (50%) exhibited congenitally missing teeth in the mandibular arch (n = 10). The side of congenitally missing teeth showed 100% concordance with the side affected by PRS (n = 6/6).
Among the three patients with mild PRS, only patient #8 (n = 1/3, 33.3%) showed two missing teeth in the maxillary arch. However, all of the two patients with moderate PRS (n = 2/2, 100%) and three of the five patients (66.7%) showed congenitally missing teeth. It can be stated that patients with moderate and severe PRS tended to have more congenitally missing teeth than did those with mild PRS.
In terms of distribution of congenitally missing teeth, the prevalence of congenital missing premolars was higher than that of missing anterior teeth and molars in the maxillary arch (nine premolars vs. five anterior teeth and five molars). However, the prevalence of congenital missing anterior teeth and premolars was higher than that of congenital missing molars in the mandibular arch (four anterior teeth and four premolars vs. two molars).
 | Figure 3Treatment outcome of Parry–Romberg syndrome (PRS) patients according to the dental phenotype. A, Patient #8 (left side involvement) had congenital missing of #23 and #25 (x-mark), impaction and short root of #24 (arrow), and prolonged retention of #63 and #65. After extraction of #65, #24 was aligned using forced eruption with fixed orthodontic treatment. #63 was maintained for substitution of #23 and spaces were created on the mesial and distal sides of #63 to compensate for the absence of #25. B, Patient #6 (right side involvement) had multiple congenital missing of #12, #14, #16, #17, #41, #44–#47 (x-mark) and microdontia with short root of #13 and #15 (arrow). She was treated with orthognathic surgery with fixed orthodontic treatment. Then, multiple-unit bridges (#11-X-X-13-15 and #31-X-42-43-X) were fabricated; dental implants could not be placed because of severe atrophy of the alveolar bone. C, Patient #9 (left side involvement) had multiple congenital missing of #21–#27, #31, #32, #34, and #35 (x-mark). She was treated with orthognathic surgery with fixed orthodontic treatment. Then, congenitally missing teeth were replaced by retained deciduous teeth and dental implant prosthesis. D, Patient #7 (left side involvement) had dilacerated root of #22 (arrow). It was aligned at an appropriate location without side effect such as root resorption. 
|
Table 2
Distribution of the number and location of congenitally missing tooth and tooth with short root, and concordance between their side of occurrence and the side affected by Parry–Romberg syndrome (PRS), according to the PRS severity index
|
Phenotype |
PRS
severity index |
Patient |
Affected
side |
Existence in the maxillary and mandibular arches |
Maxilla |
|
Mandible |
Match
with
PRS side
involvement |
|
|
|
Existence in the maxillary arches |
Incisor/canine |
Premolar |
Molar |
Sum |
Existence in the mandibular arches |
Incisor/canine |
Premolar |
Molar |
Sum |
Congenitally
missing
tooth |
Mild |
#1 |
Right |
× |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#4 |
Right |
× |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#8 |
Left |
○ |
○ |
#23 |
#25 |
|
2 |
|
|
|
|
|
|
○ |
|
Moderate |
#3 |
Left |
○ |
○ |
|
#24,25 |
#27 |
3 |
|
|
|
|
|
|
○ |
|
|
#5 |
Right |
○ |
○ |
|
#15 |
|
1 |
|
○ |
#42 |
|
|
1 |
○ |
|
Severe |
#2 |
Right |
○ |
○ |
|
#14,24 |
|
2 |
|
|
|
|
|
|
○ |
|
|
#6 |
Right |
○ |
○ |
#12 |
#14 |
#16,17 |
4 |
|
○ |
#41 |
#44,45 |
#46,47 |
5 |
○ |
|
|
#7 |
Left |
× |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#9 |
Left |
○ |
○ |
#21,22,23 |
#24,25 |
#26,27 |
7 |
|
○ |
#31,32 |
#34,35 |
|
4 |
○ |
|
|
#10 |
Left |
× |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60%
(n = 6/10) |
100%
(n = 6/6) |
5 |
9 |
5 |
19 |
|
50%(n = 3/6) |
4 |
4 |
2 |
10 |
100%
(n = 6/6) |
Tooth with
short root |
Mild |
#1 |
Right |
× |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#4 |
Right |
× |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#8 |
Left |
○ |
○ |
|
#24 |
|
1 |
|
|
|
|
|
|
○ |
|
Moderate |
#3 |
Left |
○ |
○ |
#23 |
|
|
1 |
|
|
|
|
|
|
○ |
|
|
#5 |
Right |
○ |
○ |
#13 |
#14 |
#16,17 |
4 |
|
○ |
|
#44,45 |
|
2 |
○ |
|
Severe |
#2 |
Right |
○ |
○ |
|
|
#17 |
1 |
|
|
|
|
|
|
○ |
|
|
#6 |
Right |
○ |
○ |
#13 |
#15 |
|
2 |
|
|
|
|
|
|
○ |
|
|
#7 |
Left |
× |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#9 |
Left |
× |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#10 |
Left |
○ |
○ |
|
#24,25 |
#27 |
3 |
|
○ |
|
#34,35 |
#37 |
3 |
○ |
|
|
|
|
60%
(n = 6/10) |
100%
(n = 6/6) |
3 |
5 |
4 |
12 |
|
33%(n = 2/6) |
0 |
4 |
1 |
5 |
100%
(n = 6/6) |

Occurrence of short root (Table 2 and Figure 3)
Tooth with short root was observed in six patients (n = 6/10, 60%; six patients with 12 teeth in the maxillary arch and two patients with five teeth in the mandibular arch). The side of occurrence showed 100% concordance with the side of PRS involvement (n = 6/6, 100%).
Among the three patients with mild PRS, only patient #8 (n = 1/3, 33.3%) showed one short root in the maxillary arch. However, all of the two patients with moderate PRS (n = 2/2, 100%) and three of the five patients with severe PRS (n = 3/5, 66.7%) showed teeth with short roots. It can be stated that patients with moderate and severe PRS tended to have more teeth with short roots than did those with mild PRS.
In the maxillary arch, the prevalence of tooth with short root was similar (3 anterior teeth, 5 premolars, and 4 molars). However, prevalence of short root in the premolars was higher than that of the anterior teeth and molars in the mandibular arch (4 premolars vs. 0 anterior tooth and 1 molar).
Average number of congenitally missing tooth and short root (Table 3)
Since congenitally missing tooth and short root are two major dental phenotypes which can influence the occlusal function, aesthetics, space problem, and orthodontic anchorage, we combined these two phenotypes to identify the characteristics of PRS. The sum of the average numbers of missing tooth and short root was 1.0 (0.67 + 0.33) in mild PRS cases, 6.0 (2.5 + 3.5) in moderate PRS cases, and 6.2 (4.4 + 1.8) in severe PRS cases.
Table 3
Sum of the average number of congenitally missing tooth and tooth with short root according to the Parry–Romberg syndrome (PRS) severity index
|
PRS severity index |
Patient |
Congenitally missing tooth |
Tooth with short root |
Sum of congenitally
missing tooth and
tooth with short root |
|
Mild |
#1 |
0 |
0 |
0 |
|
#4 |
0 |
0 |
0 |
|
#8 |
2 |
1 |
3 |
|
Mean |
0.67 (n = 2/3) |
0.33 (n = 1/3) |
1.0 (n = 3/3) |
|
Moderate |
#3 |
3 |
1 |
4 |
|
#5 |
2 |
6 |
8 |
|
Mean |
2.5 (n = 5/2) |
3.5 (n = 7/2) |
6.0 (n = 12/2) |
|
Severe |
#2 |
2 |
1 |
3 |
|
#6 |
9 |
2 |
11 |
|
#7 |
0 |
0 |
0 |
|
#9 |
11 |
0 |
11 |
|
#10 |
0 |
6 |
6 |
|
Mean |
4.4 (n = 22/5) |
1.8 (n = 9/5) |
6.2 (n = 31/5) |

Occurrence of microdontia, delayed eruption/impacted tooth, and dilacerated root (Table 4 and Figure 3)
Microdontia was observed in four patients (40%; 12 teeth, prevalent at the maxillary arch [10 microdontia in the maxillary arch vs. 2 microdontia in the mandibular arch]). Delayed eruption/impacted tooth was observed in only two patients (20%; 3 teeth, prevalent at the maxillary arch [all in the maxillary arch]). Tooth with dilacerated root was also observed in only two patients (20%; 2 teeth, one arch in the maxillary and mandibular arches). The side of occurrence of these dental problems showed 100% concordance with the side of PRS involvement (n = 4/4, n = 2/2, n = 2/2, all 100%).
Among the three patients with mild PRS, two patients (n = 2/3, 66.7%) showed these dental problems. All of the two patients with moderate showed microdontia (n = 2/2, 100%). Among the five patients with severe PRS, three patients showed these dental problems (n = 3/5, 66.7%). Since there was no tendency of increase of these dental problems from the mild, moderate to severe PRS, the prevalence of microdontia, delayed eruption/impacted tooth, and dilacerated root might not to be associated with the severity of PRS.
Table 4
Distribution of microdontia, delayed eruption/impacted tooth, and tooth with dilacerated root according to the Parry–Romberg syndrome (PRS) severity index
|
PRS severity index |
Patient |
Affected side |
Microdontia |
|
Delayed eruption/impacted tooth |
|
Tooth with
dilacerated root |
Match with side involvement |
|
|
|
|
Existence |
Number |
Existence |
Number |
Existence |
Number |
|
Mild |
#1 |
Right |
× |
|
|
× |
|
|
○ (#43) |
1 |
○ |
|
#4 |
Right |
× |
|
|
× |
|
|
× |
|
|
|
#8 |
Left |
○ (#24,26) |
2 |
|
○ (#24) |
1 |
|
× |
|
○ |
|
Moderate |
#3 |
Left |
○ (#23,26) |
2 |
|
× |
|
|
× |
|
○ |
|
#5 |
Right |
○ (#13,14,16,17,44,45) |
6 |
|
× |
|
|
× |
|
○ |
|
Severe |
#2 |
Right |
× |
|
|
○ (#13,17) |
2 |
|
× |
|
○ |
|
#6 |
Right |
○ (#13,15) |
2 |
|
× |
|
|
× |
|
○ |
|
#7 |
Left |
× |
|
|
× |
|
|
○ (#22) |
1 |
○ |
|
#9 |
Left |
× |
|
|
× |
|
|
× |
|
|
|
#10 |
Left |
× |
|
|
× |
|
|
× |
|
|
|
n = 4 (40%) |
n = 2 (20%) |
n = 2 (20%) |
n = 7/7 (100%) |

Distribution of the types of Tx-Mod
Among the patients with mild PRS, patient #4 was followed-up with growth observation due to mild atrophy and facial asymmetry at initial visit of preadolescent age (Tx-Mod-1); Patient #8 was treated with fixed orthodontic treatment only (Tx-Mod-3); and patient #1 was treated with fixed orthodontic treatment (Tx-Mod-3) and grafting (Tx-Mod-5).
All of the patients with moderate PRS underwent growth observation due to a definite need of surgical intervention (Tx-Mod-4) because they visited the clinic at preadolescent age.
In the patients with severe PRS, although orthognathic surgery with fixed orthodontic treatment (Tx-Mod-6) was planned for patient #2, he gave up the treatment; After growth observation for surgery (Tx-Mod-4), patient #6 was treated with grafting (Tx-Mod-5) and orthognathic surgery with fixed orthodontic treatment (Tx-Mod-6); Patient #7 was consecutively treated with unilateral functional appliance (Tx-Mod-2), fixed orthodontic treatment (Tx-Mod-3) and grafting (Tx-Mod-5); Patient #9 was treated with grafting (Tx-Mod-5) and orthognathic surgery with fixed orthodontic treatment (Tx-Mod-6); and patient #10 was followed-up with growth observation for surgery (Tx-Mod-4) because of initial visit at a high school age.
Go to :

DISCUSSION
Compared to previous case reports and review article,
8,10,13-21 the present study has some advantages as follows: First, longitudinal data of 10 unrelated Korean patients with PRS could provide primary information on the dental phenotypes and Tx-Mod types. Second, instead of subjective assessment of facial atrophy, we devised an objective index to determine the PRS severity using the numbers of atrophy-involved area and asymmetry-involved item. Third, we evaluated the dental phenotypes, including congenitally missing teeth, short root, microdontia, dilacerated root, and delayed eruption/impacted tooth according to the novel PRS severity index. Fourth, we described the Tx-Mod types according to the novel PRS severity index.
Clinical implications for the dental phenotypes in patients with PRS
If PRS becomes apparent before the age of 10 or in the early first decade of life, the active stage of atrophy may coincide with the stage of tooth germ formation, development of crown and root, and eruption of the permanent teeth. Therefore, dental problems in PRS patients could be diverse, including congenitally missing, short root, microdontia, delayed eruption/impacted teeth, and dilacerated root (
Tables 2 and
4).
We summarized the dental phenotypes in our PRS patients and compared our findings with those of the previous case reports and review (
Table 5).
8,10,11,13-16,18-21 First, congenitally missing teeth were reported by Urban et al.
16 and Grippaudo et al.
20 in their case reports (
Table 5). In the present study, the prevalence of congenital missing teeth was 60%, and the side of occurrence matched with the side of PRS involvement in all six patients (
Table 2). Moreover, the prevalence of congenitally missing tooth was higher in the maxillary arch than the mandibular arch (six patients with 19 missing teeth vs. three patients with 10 missing teeth,
Table 2). The mechanism underlying the congenitally missing of teeth in these patients might be related with the ectodermal origin of teeth. However, it is necessary to investigate the exact pathophysiology of congenital missing tooth in these patients. Second, numerous case reports have reported the existence of short root in patients with PRS (
Table 5).
8,13-15,18,20,21 In the present study, tooth with short root was observed in 60% of the patients and the side of occurrence matched with the side of PRS involvement in all patients (
Table 2). It was also more prevalent in the maxillary arch than in the mandibular arch (six patients with 12 teeth vs. two patients with five teeth,
Table 2). Since the sum of the average numbers of missing tooth and tooth with short root increased from mild PRS (1.0) to moderate PRS and severe PRS (6.0 and 6.2,
Table 3), it can be stated that our novel PRS severity index can describe the susceptibility of PRS patients to congenital missing tooth and short root. Third, although the side of occurrence of microdontia, impacted tooth and dilacerated root matched with the side of PRS involvement (n = 4/4, n = 2/2, n = 2/2, all 100%,
Table 2), the prevalence of these problems was relatively low (microdontia, 40%; impacted tooth, 20%; and dilacerated root, 20%), with no evidence of association between their prevalence and the severity of PRS (
Table 4). Therefore, it can be stated that congenitally missing tooth and short root are presentative dental phenotypes in patients with moderate and severe PRS.
Table 5
Comparison between the results of previous studies and those of the present study on Parry–Romberg syndrome
|
Author (year) |
Study
design |
Sample (race, number) |
Root |
Microdontia |
Delayed eruption/impacted tooth |
Congenital missing |
|
|
Short root (incomplete root development) |
Dilacerated root |
|
Foster13 (1979) |
Case report |
Caucasian(n = 1) |
Present
#44,45,46,47 |
Not mentioned |
Not mentioned |
Not mentioned |
Not mentioned |
Fayad and
Steffensen14
(1994) |
Case report |
Hispanic(n = 1) |
Present
#12,42 |
Not mentioned |
Not mentioned |
Not mentioned |
Not mentioned |
Mazzeo
et al.15 (1995) |
Case report |
Hispanic(n = 1) |
Present
#23,36,37 |
Not mentioned |
Not mentioned |
Not mentioned |
Not mentioned |
Urban
et al.16 (1996) |
Case report |
Hispanic(n = 1) |
Not mentioned |
Not mentioned |
Not mentioned |
Not mentioned |
Mentioned(a lack of the second molar) |
Colquhoun
et al.18 (2000) |
Case report |
Caucasian(n = 1) |
Present
Generalized shortening of all permanent incisor, premolar, and lower canine |
Not mentioned |
Not mentioned |
Present
#24,25,27,37 |
Not mentioned |
Grippaudo
et al.20 (2004) |
Case report |
Caucasian(n = 2) |
Present
Patient #1: malformed(first molar and first premolar) roots
Patient #2: root reduction with several teeth (#14, 16,17,31,41,42,44) |
Not mentioned |
Not mentioned |
Present
Patient #1: the teeth on the left maxillary side were less erupted |
Present
Patient #1: agenesis of the second premolar, #15,25,35,45
Patient #2: #15,36 |
O'Flynn and
Kinirons8 (2006) |
Case report |
Caucasian(n = 1) |
Present
#24,25,26,27,34,35,
36,37 |
Present
#24,34,45 |
Not mentioned |
Present
#27,37 |
Not mentioned |
de Vasconcelos
Carvalho
et al.11 (2010) |
Case report |
Brazilian(n = 1) |
Not mentioned |
Not mentioned |
Not mentioned |
Not mentioned |
Not mentioned |
You and Baik21
(2011) |
Case report |
Korean(n = 1) |
Present
#34,35 |
Not mentioned |
Not mentioned |
Present
Posterior open bite on the left side due to delayed tooth eruption |
Not mentioned |
Dixit
et al.19 (2016) |
Case report |
Dravidian, India (n = 2) |
Not mentioned |
Not mentioned |
Mentioned(relative microdontia on the left side) |
Not mentioned |
Not mentioned |
Al-Aizari
et al.10 (2015) |
Review of 14
papers |
- |
Mentioned |
Not mentioned |
Not mentioned |
Mentioned |
Mentioned |
This study
(2019) |
Retrospective
chart review |
Korean (n = 10) |
Present(n = 6/10) |
Present(n = 2/10) |
Present(n = 4/10) |
Present(n = 2/10) |
Present(n = 6/10) |

In terms of orthodontic treatment strategy for solving the dental problems in patients with PRS (
Figure 3), congenitally missing tooth can be managed according to the presence of retained deciduous tooth. If the deciduous tooth is retained, it can be maintained as substitute for the congenitally missing tooth. However, if the deciduous tooth is not retained, the space for the congenitally missing tooth needs to be created or maintained. Then, prosthodontic treatment including dental implant or bridge can be applied according to the degree of alveolar bone atrophy. For microdontia, it is necessary to measure the mesiodistal width of the same tooth in the unaffected side. Adequate space for resin or prosthodontic treatment is required on the mesial and distal sides of microdontia. Therefore, in case of congenitally missing tooth or microdontia, careful communication with a prosthodontist from the diagnosis and treatment planning stage to the end of orthodontic treatment period is necessary. Since tooth with short or dilacerated root might have an increased possibility of external root resorption by orthodontic force, they should be carefully monitored during orthodontic treatment, especially during torque control and root movement. Frequent radiographic evaluation might be necessary for evaluation of the root condition. For delayed eruption/impacted tooth, the eruption status of the same tooth on the unaffected side should be checked, and operculectomy or window opening for forced eruption of that tooth should be implemented at an appropriate time.
Clinical considerations of Tx-Mod in patients with PRS
If patients with PRS seek consultation at a pre-adolescent age or an adolescent age and do not show significant atrophy and facial asymmetry, growth observation (Tx-Mod-1) can be recommended. If PRS patients visit before completion of growth and show significant atrophy and facial asymmetry, growth observation for surgery (Tx-Mod-4) is the first-of-choice of treatment.
In patients with mild-to-moderate PRS, active use of unilateral functional appliance (Tx-Mod-2) and fixed orthodontic treatment (Tx-Mod-3) might result in favorable changes in the dentoalveolar and skeletal problems during the adolescent growth period. In cases of moderate and severe PRS, orthodontists should determine whether the dental problems and facial asymmetry can be treated with an orthodontic or a surgical approach. In that case, orthodontists can focus on restoring or maintaining the arch dimension and vertical height during orthodontic treatment. Diverse combinations of Tx-Mod including unilateral functional appliance (Tx-Mod-2), fixed orthodontic treatment (Tx-Mod-3), growth observation for surgery (Tx-Mod-4), grafting (Tx-Mod-5), or orthognathic surgery with orthodontic treatment (Tx-Mod-6) can be used to restore the soft tissue deformities and reconstruct the skeletal framework according to the growth stage and degree of atrophy and facial asymmetry.
Although the present study might provide basic information on the dental phenotypes and Tx-Mod types in PRS patients, it is necessary to perform a prospective study with a large sample size from nationwide multi-centers in future studies.
Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download