INTRODUCTION
MATERIALS AND METHODS
Animals
Orthodontic rotational movement
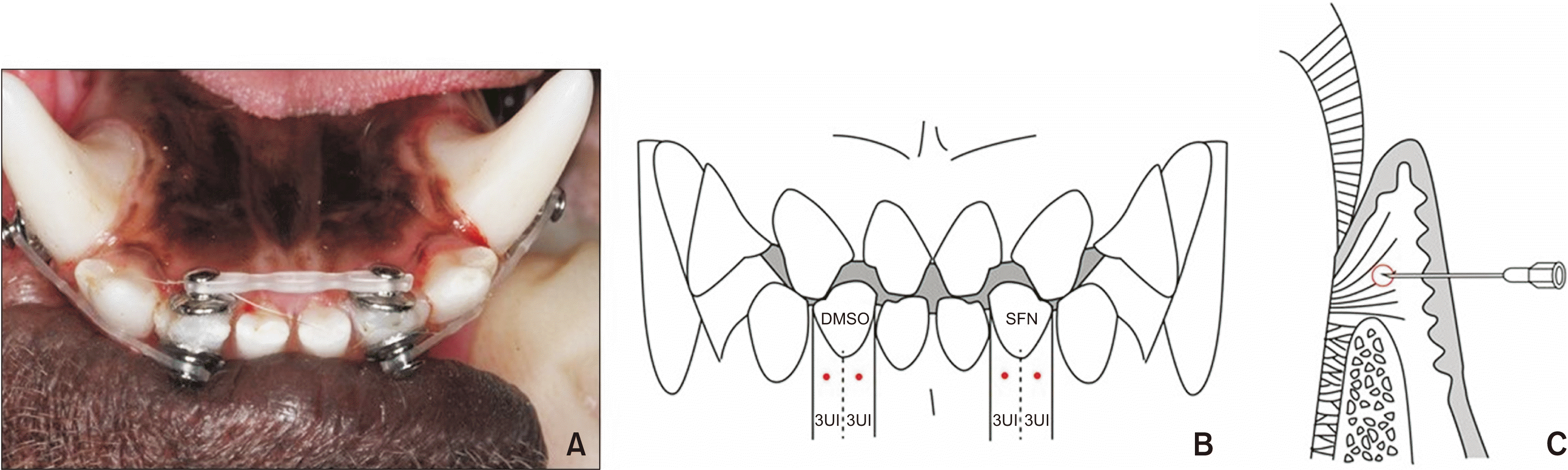 | Figure 1
A, Occlusal view of a beagle’s lower teeth and orthodontic buttons with elastic chains. Mandibular lateral incisors (I2 teeth) of beagle dogs were rotated for 6 weeks by applying rotational couple force using a power chain. B, C, The needle injection points of gingiva of experimental teeth. After the needle was inserted at right angles to the attached gingiva area located directly below the boundary between free gingiva and attached gingiva, vertically, and at both centers of half of the buccal gingiva of the experimental teeth, horizontally, sulforaphane (SFN) and dimethylsulfoxide (DMSO) were injected into each side of 3 units (UI) (B, frontal view; C, sagittal view). |
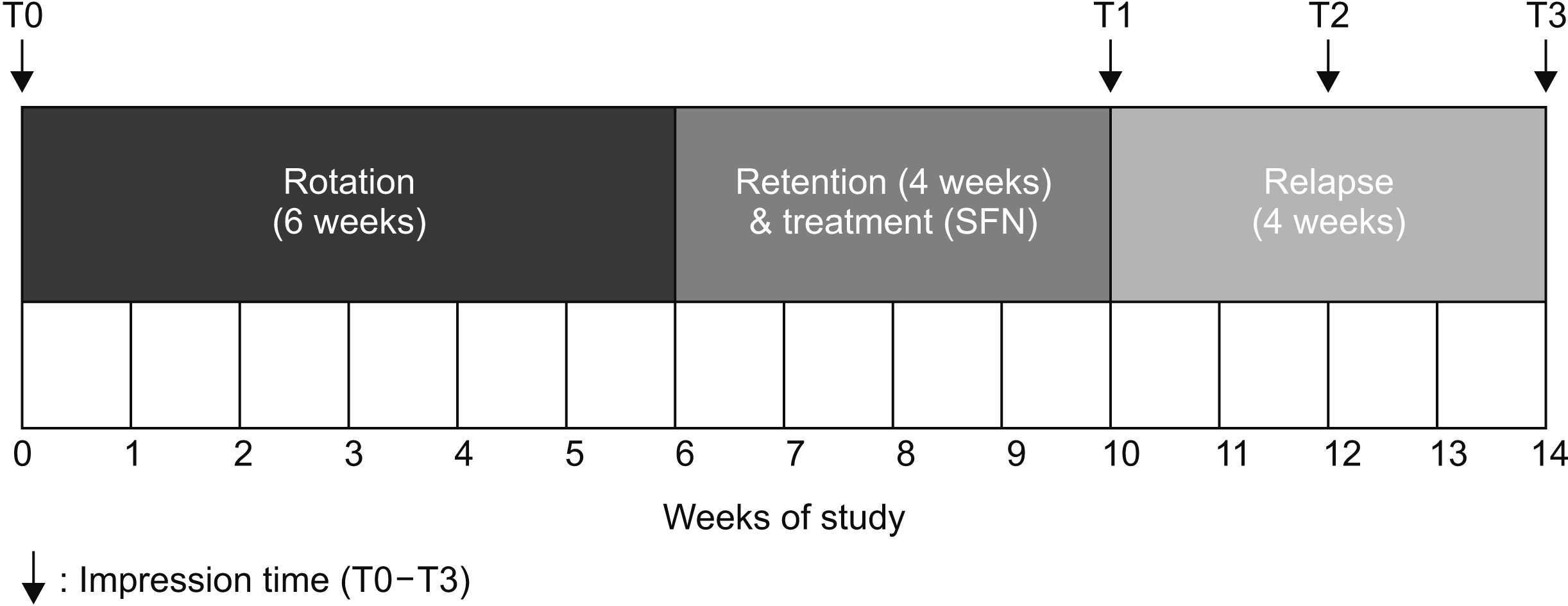 | Figure 2Schema. The experimental schedule for the beagle dogs. The experimental teeth were rotated by applying rotational couple force with a power chain for 6 weeks. During a retention period of 4 weeks, SFN (experimental group) and DMSO (control group) were injected into the supra-alveolar gingiva of the buccal side of the rotated teeth once per week, four times total. One week after the last injection, the appliances were removed to allow relapse of the rotated teeth. An intraoral impression was obtained to measure the extent of tooth rotation and relapse.
SFN, Sulforaphane; DMSO, dimethylsulfoxide; T0, initial; T1, immediately after retention and SFN treatment for 4 weeks; T2, after relapse for 2 weeks; T3, after relapse for 4 weeks.
|
Model analysis
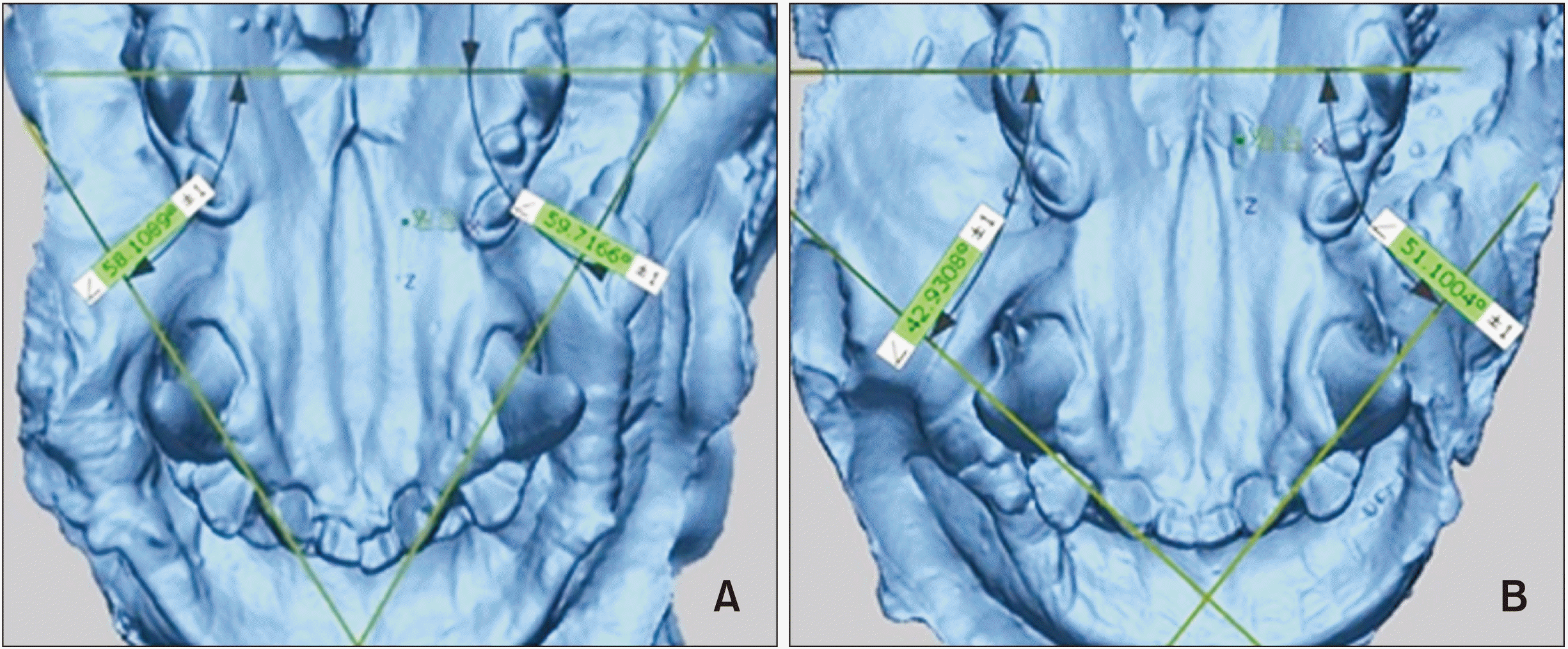 | Figure 3The extent of tooth rotation and relapse. Measurements were made using the Geomagic Control XTM (3D SYSTEMS, Seoul, Korea) software. The degree of rotation and amount of relapse after 2 and 4 weeks, and percentage of relapse after 2 and 4 weeks were calculated. A, Scanning image of T1 model from beagle no. 1. B, Scanning image of T2 model from beagle no. 1.
T1, Immediately after retention and SFN treatment for 4 weeks; T2, after relapse for 2 weeks.
|
Real-time quantitative polymerase chain reaction
Table 1
Histologic examination using Masson’s trichrome
Statistical analysis
RESULTS
Model analysis - sulforaphane treatment reduces the amount and percentage of orthodontic rotation relapse
Table 2
| Group | Degree of rotation (°) | Amount of relapse (°) | Percentage of relapse (%) | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| After 2 weeks | After 4 weeks | After 2 weeks | After 4 weeks | |||
| Control | 21.40 ± 5.95 | 13.19 ± 3.88 | 16.96 ± 5.55 | 64.57 ± 18.86 | 81.09 ± 18.80 | |
| Experimental | 23.32 ± 2.57 | 6.71 ± 2.99* | 9.19 ± 3.31*,† | 28.01 ± 10.68* | 38.85 ± 11.85*,† | |
The symbol (*) indicates statistical significance between the control group and experimental group based on intergroup comparisons (*p < 0.05, paired t-test). The symbol (†) indicates statistical significance of the mean amount and percentage of rotational relapse between 2 and 4 weeks after the retention period based on intragroup comparisons (†p < 0.05, paired t-test).
Real-time polymerase chain reaction analysis - sulforaphane treatment alters the expression of genes associated with gingival elasticity in beagle dogs
 | Figure 4Relative mRNA expression of genes encoding COL1A1, LOX, ELN, MMP 1, MMP 12, and TIMP 1 in gingival tissue of beagle dogs after sulforaphane treatment. COL1A1 (A), LOX (B), ELN (C), MMP 1 (D), MMP 12 (E), and TIMP 1 (F) mRNA levels in gingival tissue were quantified by real-time polymerase chain reaction. The symbol (*) indicates statistical significance between the control group and experimental group (*p < 0.05, Wilcoxon signed rank test).
DMSO, Dimethylsulfoxide; COL1A1, collagen type I alpha 1; LOX, lysyl oxidase; ELN, elastin; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
|
Histologic examinations
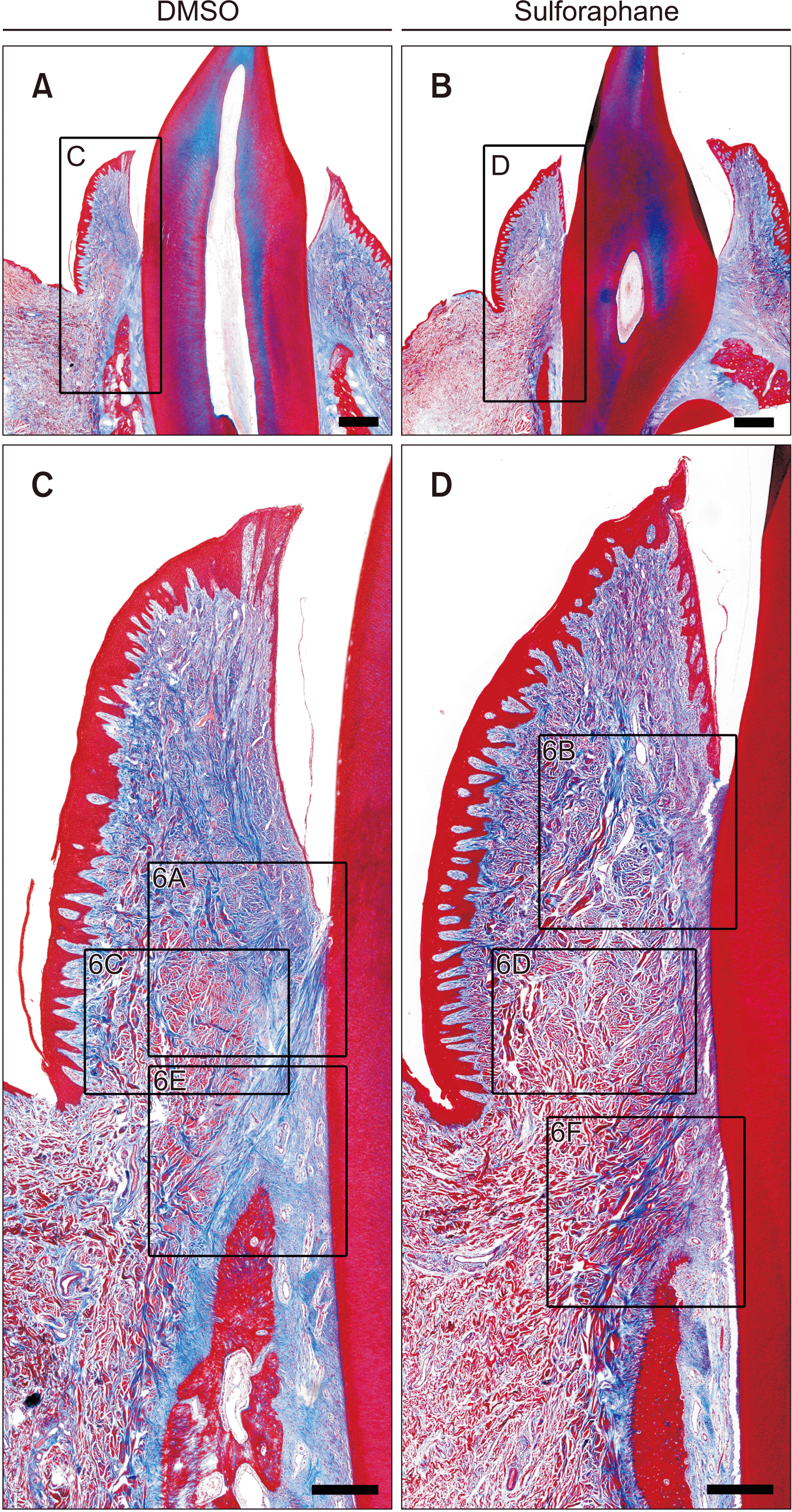 | Figure 5Histologic appearance of collagen fibers in buccal supra-alveolar gingiva. Massonʼs trichome staining of the buccal-lingual longitudinal sections of the lower lateral incisors of beagle dogs injected with either dimethylsulfoxide (DMSO) or sulforaphane (SFN) for 4 weeks. The control and experimental samples were from same beagle subject. Collagen fibers bundles were longer and more connected in the DMSO-treated group than in the SFN-treated group. A, C, DMSO group. B, D, SFN group. Scale bar = 1 mm (A, B), 400 μm (C, D). Higher magnification views of the box areas can be seen Figure 6. |
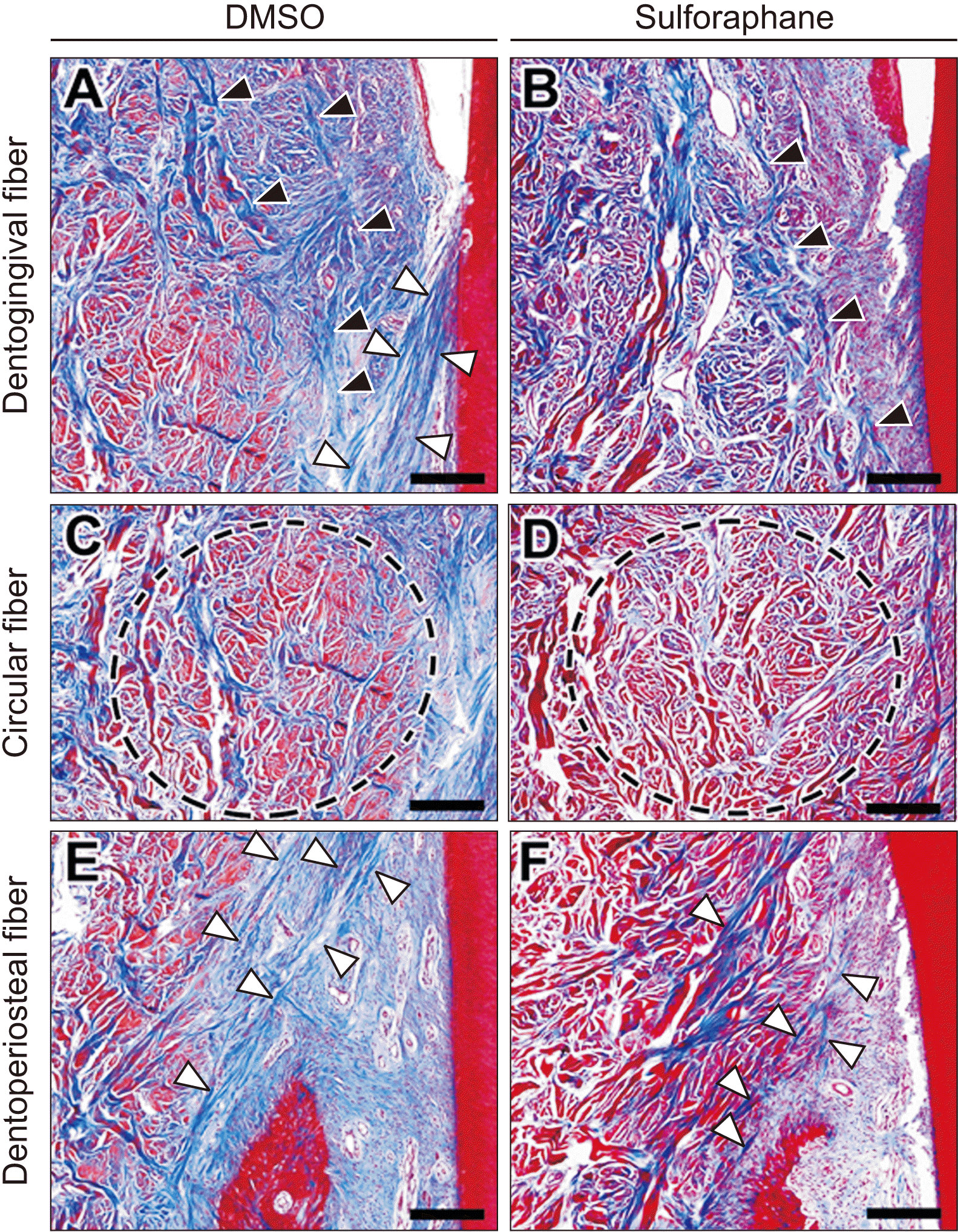 | Figure 6Morphology and arrangement of principal gingival fibers in the buccal supra-alveolar gingiva. The higher magnification views of dentogingival fibers (black arrows indicate dentogingival fibers) and dentoperiosteal fibers (white arrows indicate dentoperiosteal fibers) showed that collagen fiber bundles connected to form the principal gingival fibers were irregularly and not well connected in the sulforaphane (SFN)-treated group compared to the dimethylsulfoxide (DMSO)-treated group. Circular fibers (indicated in black circle dotted lines) of the SFN-treated group were arranged in a thinner and less compact manner compared to the DMSO-treated group. A, C, E, DMSO group. B, D, F, SFN group. Scale bar = 200 μm. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download