Abstract
Melatonin and cortisol are clinically important for diagnosing sleep and mood disorders. We developed and validated a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for simultaneous measurement of salivary melatonin and cortisol concentrations according to the Clinical and Laboratory Standards Institute guidelines. Additionally, we compared the LC-MS/MS assay with immunoassays, ELISA (Direct Salivary Melatonin Elisa EK-DSM, Bühlmann Laboratories AG, Schönenbuch, Switzerland) and electrochemiluminescence immunoassay (Cortisol II, Roche, Mannheim, Germany), using 121 saliva samples. The LC-MS/MS assay exhibited good performance in terms of linearity, precision, accuracy, limit of detection, lower limit of quantification, extraction recovery, carry-over, and matrix effect. The LC-MS/MS assay and immunoassays showed strong correlation (Pearson’s r=0.910 for melatonin, r=0.955 for cortisol), but demonstrated a significant mean bias of 23.2% (range 54.0–143.7%) for melatonin and 48.9% (range 59.7–184.7%) for cortisol. Our LC-MS/MS assay provided more sensitive and reliable salivary melatonin and cortisol quantification results compared with immunoassays.
Melatonin, a hormone primarily secreted by the pineal gland, is clinically important for the diagnosis of sleep and mood disorders and affects the circadian rhythm and many other physiological functions [1]. Cortisol, a steroid hormone secreted by the adrenal gland, has been associated with physical and emotional stress as well as the circadian rhythm [2]. As studies examining the sleep cycle often require consecutive samples collected over a 24-hour period [3], saliva samples are more advantageous than blood samples, because they do not require venipuncture at any time point. In addition, salivary concentrations of melatonin and cortisol are well-correlated with plasma concentrations, suggesting the possible use of salivary samples as a surrogate for plasma samples [4, 5].
Hormone measurement in routine clinical laboratories relies largely on immunoassays, owing to advantages such as low cost and technical ease [6]. However, the cost and required sample volume increase when multiple analytes are measured in a single sample, as each analyte is measured on a separate immunoassay platform. Additional drawbacks of immunoassay include cross-reactivity and insufficient sensitivity for low-concentration target hormones. Melatonin assays need to be sensitive enough to detect low concentrations (<8 pmol/L) for determining dim light melatonin onset in circadian phase studies [7]. Furthermore, the late-night salivary cortisol concentration is reported to be as low as 3 nmol/L in normal control groups, which is close to current immunoassay detection limits [8].
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) has been introduced as a sensitive and specific assay, with the advantage of multiplexing for simultaneous measurement of melatonin and cortisol concentrations [3, 9]. The measurement of salivary melatonin and cortisol concentrations using LC-MS/MS assay with different extraction methods has been reported [10–12]. However, a previous assay comparison study on both salivary melatonin and cortisol concentrations was based on only eight spiked saliva samples, which is too small a group for proper comparison [13]. We developed and validated an LC-MS/MS assay for simultaneous measurement of salivary melatonin and cortisol concentrations, according to the Clinical and Laboratory Standards Institute guidelines C62-A [14]. In addition, we compared our LC-MS/MS assay with commercial immunoassays, including an enzyme-linked immunosorbent assay (ELISA, Direct Salivary Melatonin Elisa EK-DSM, Bühlmann Laboratories AG, Schönenbuch, Switzerland) for melatonin and an electrochemiluminescence immunoassay (ECLIA, Cortisol II, Roche, Mannheim, Germany) for cortisol, using 121 salivary samples.
Twenty healthy participants with self-reported sleep onset occurring between 23:00 and 00:00 and a sleep length of 7–9 hours without sleep complaints or sleep disorders were enrolled prospectively. Saliva samples were collected between 18:00 and 00:00 hours, consecutively, by chewing on Parafilm (Bemis Company Inc., Neenah, WI, USA) and drooling into a conical polypropylene tube. All collected samples were more than 2 mL in volume. Samples were frozen and stored at −20°C until delivery to the laboratory at Samsung Medical Center, Seoul, Korea, for analysis. The study was conducted in February 2018, with an approval by the Samsung Medical Center Institutional Review Board (IRB No. 2017-08-125), and informed consent was obtained from all participants.
Stock solutions were prepared by dissolving melatonin or cortisol (Sigma Aldrich, St. Louis, MO, USA) in methanol (Burdick & Jackson, Muskegon, MI, USA) and were frozen and stored at −80°C until use. The stock solutions were diluted with 10% (v/v) methanol to prepare the calibrators at five concentrations (2.2, 21.5, 107.5, 215, and 430 pmol/L for melatonin and 0.14, 1.38, 6.9, 13.8, and 27.6 nmol/L for cortisol) and internal standard (IS) solutions (melatonin-d4, 2,150 pmol/L and cortisol-d4, 138 nmol/L). Quality control (QC) samples were prepared at three concentrations (8.6, 86, and 344 pmol/L for melatonin and 0.55, 5.52, and 22.08 nmol/L for cortisol) using the same procedure as for the calibrators but with a different batch of reagents.
For the LC-MS/MS assay, 300 μL of saliva, 20 μL of IS solution, and 1,000 μL of methyl tert-butyl ether were added to Eppendorf tubes. The tubes were sealed and vortexed for 30 min and then centrifuged at 20,600×g for 10 minutes. Next, 930 μL of the supernatant was transferred to the wells of a 2.0 mL polypropylene 96-deep well plate and dried with a microplate evaporator (Evaporex EVX-96, Apricot Designs, Monrovia, CA, USA). Then, 100 μL of 20% (v/v) methanol was added to the plate and mixed for 30 minutes, and the plate was transferred to the LC-MS/MS spectrometer.
Samples were injected and analyzed on an Agilent 6,490 tandem mass spectrometer equipped with an Agilent 1,260 high-performance liquid chromatography (HPLC) system (Agilent Technologies, Santa Clara, CA, USA) and a C18 2.1×50 mm 2.6 μm Kinetex column (Phenomenex, Torrance, CA, USA). The injection volume was 20 μL. The mobile phase consisted of 2-mmol/L ammonium acetate in deionized water (A) and 0.1% (v/v) formic acid in acetonitrile (B). The gradient conditions are detailed in Supplemental Data Table S1. The total run time was 6 min, with a flow rate of 250 μL/min.
The mass spectrometer was equipped with a jet stream electrospray ionization source operating in positive ion mode. Quantification was performed using the peak area ratio of melatonin and cortisol to IS with MassHunter Workstation software (version B.06, Agilent Technologies). Multiple reaction monitoring (MRM) transition, collision energy, cell accelerator voltage, and retention time are provided in Supplemental Data Table S2.
The calibration curves were linear over the melatonin (2.15–430 pmol/L) and cortisol (0.14–27.59 nmol/L) calibration ranges, with a correlation coefficient r>0.99 for both melatonin and cortisol (Table 1). The limit of detection (LOD) was determined as the lowest concentration assayed with a signal-to-noise ratio (SNR)>3. The lower limit of quantification (LLOQ) was determined as the lowest concentration assayed with an SNR>10, a coefficient of variation (CV) <20%, and a bias <15% in replicate assays [14]. The LLOQs of melatonin and cortisol were 2.15 pmol/L and 0.14 nmol/L, respectively. Accuracy was evaluated as relative recovery at three concentrations, intra-assay precision was assessed using five replicates of three QC samples each for melatonin and cortisol, and inter-assay precision was determined on 30 different assay days.
The matrix effect was evaluated by post-extraction spike methods at three concentrations as the ratio of the analyte peak area in post-extraction spiked saliva to that in a methanol solution, multiplied by 100. Carry-over was evaluated with a blank sample after running the highest concentration calibrator. Extraction recoveries ranged from 100.9% to 102.6% for melatonin and from 100.1% to 103.7% for cortisol. No significant matrix effects were observed, with values of 92.1–97.7% for melatonin and 98.8–99.0% for cortisol. No significant carry-over was observed.
Salivary melatonin and cortisol concentrations were also measured by ELISA (Direct Salivary Melatonin ELISA EK-DSM, Bühlmann Laboratories AG, Schönenbuch, Switzerland) and ECLIA (Cortisol II, Roche, Mannheim, Germany), respectively. Based on the details provided in the kits, the LLOQs of ELISA and ECLIA were 6.9 pmol/L and 3.0 nmol/L, respectively. The required sample volume was 200 and 500 μL for salivary melatonin and cortisol concentration measurements, respectively. Passing-Bablok regression, Pearson’s correlation, and Bland-Altman plots were used for assay comparison. The data were analyzed using the R software version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Salivary melatonin concentrations measured by ELISA were higher than those measured by the LC-MS/MS assay. Bland-Altman analysis showed a bias of 7.70 pmol/L (95% CI: 4.64–10.79 pmol/L), corresponding to a percent mean bias of 23.2% (95% CI: 14.1–32.3%; Fig. 1). Similarly, salivary cortisol concentrations measured by ECLIA were higher than those measured by the LC-MS/MS assay. Bland-Altman analysis showed a bias of 1.68 nmol/L (95% CI: 1.02–2.32 nmol/L), corresponding to a percent mean bias of 48.9% (95% CI: 37.4–60.4%; Fig. 1).
We plotted the salivary melatonin and cortisol concentrations in consecutive 6-hr samples from two patients, measured by both the LC-MS/MS assay and immunoassays (Fig. 2). For the cortisol immunoassay measurements, concentrations below the LOD were reported and plotted as 1.49 nmol/L. Participant dim light melatonin onset was estimated to occur immediately after 22:00. Aberrantly high melatonin concentrations were observed at 18:00 only by the immunoassay.
The comparison of the LC-MS/MS assay with the immunoassays showed strong correlation but biased results for both salivary melatonin and cortisol concentrations, with immunoassays consistently yielding higher values. Previous comparison studies also reported that the melatonin concentrations measured by ELISA were higher than those by LC-MS/MS assay in the low melatonin concentration range [10, 13]. Notably, the opposite trend has been reported in the higher melatonin concentration range (van Faassen, et al. [10], >30 pmol/L; Jensen, et al. [13], >76 pmol/L). These results suggest that melatonin concentrations measured by ELISA is unreliable in the high melatonin concentration range.
Jensen, et al. [13] also reported higher cortisol concentrations measured by ELISA (IBL, Hamburg, Germany) compared with LC-MS/MS assay. Vogeser, et al. [15] reported well correlated ECLIA (Cortisol II, Roche) and LC-MS/MS assay results with standardized calibrators. The observed bias between ECLIA and LC-MS/MS assay in our study would be partly due to the saliva samples containing analyte concentrations below the LLOQ of ECLIA.
The serial 6-hour plots of the two samples (Fig. 2) reveal a performance advantage for LC-MS/MS assay over the immunoassays. The lower LC-MS/MS assay LLOQ for cortisol enabled better discrimination of the cortisol profile in lower concentration ranges. Of the samples assayed, 30% had values below the ECLIA LLOQ for cortisol measurement, while none were below the LC-MS/MS assay LLOQ. In addition, the immunoassays were more sensitive to interference due to the collection method [16] and cross-reacting substances. LC-MS/MS assay demonstrated less interference and provided more specificity for the analyte of interest. The melatonin profiles (measured by the different assays) illustrate the difficulties associated with accurate analyte measurement (Fig. 2). The discrepant melatonin profiles by LC-MS/MS assay and immunoassays at 18:00 (Fig. 2) were also noted in the melatonin profiles of the other participants. Normally, the melatonin concentration in the body follows a bell-shaped pattern with a single peak at night [17]. Of the measured melatonin concentrations at 18:00, the LC-MS/MS profile fits better with the normal pattern of healthy participants and is considered as the true concentration. The discrepancy could have been caused by cross-reactivity or interference from other hormones in the immunoassay. Although previous studies on the secretion of melatonin or cortisol have relied on immunoassays [18, 19], LC-MS/MS assay provided improved analytical performance, which is required for secretion studies.
In addition, while the two separate salivary melatonin and cortisol immunoassays required a total sample volume of 700 μL, simultaneous measurement of melatonin and cortisol concentrations by LC-MS/MS assay required only 300 μL of saliva, less than a half of that required for the immunoassays.
As all saliva samples were collected from healthy subjects, our comparison study was not conducted using samples from various disease states to avoid interference with the measurement results. Further study is needed to explain the discrepancy observed at certain time points between LC-MS/MS assay and immunoassays.
In conclusion, an LC-MS/MS assay for simultaneous measurement of salivary melatonin and cortisol concentrations was developed and validated. Compared with immunoassays, LC-MS/MS assay offered greater sensitivity and specificity, which are essential for both routine clinical use and research on circadian rhythms or sleep disorders.
Analyte and internal standard MS parameters
Notes
REFERENCES
1. Claustrat B, Leston J. Melatonin: physiological effects in humans. Neurochirurgie. 2015; 61:77–84.


2. Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell-Price J, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009; 94:1548–54.



3. Blair J, Adaway J, Keevil B, Ross R. Salivary cortisol and cortisone in the clinical setting. Curr Opin Endocrinol Diabetes Obes. 2017; 24:161–8.


4. Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997; 12:457–66.


5. Jung C, Greco S, Nguyen HH, Ho JT, Lewis JG, Torpy DJ, et al. Plasma, salivary and urinary cortisol levels following physiological and stress doses of hydrocortisone in normal volunteers. BMC Endocr Disord. 2014; 14:91.



6. Taylor AE, Keevil B, Huhtaniemi IT. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur J Endocrinol. 2015; 173:D1–12.

7. Kennaway DJ. A critical review of melatonin assays: past and present. J Pineal Res. 2019; 67:e12572.

8. Doi M, Sekizawa N, Tani Y, Tsuchiya K, Kouyama R, Tateno T, et al. Late-night salivary cortisol as a screening test for the diagnosis of Cushing’s syndrome in Japan. Endocr J. 2008; 55:121–6.


9. Turpeinen U, Hämäläinen E. Determination of cortisol in serum, saliva and urine. Best Pract Res Clin Endocrinol Metab. 2013; 27:795–801.


10. van Faassen M, Bischoff R, Kema IP. Relationship between plasma and salivary melatonin and cortisol investigated by LC-MS/MS. Clin Chem Lab Med. 2017; 55:1340–8.


11. Fustinoni S, Polledri E, Mercadante R. High-throughput determination of cortisol, cortisone, and melatonin in oral fluid by on-line turbulent flow liquid chromatography interfaced with liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2013; 27:1450–60.


12. Jensen MA, Hansen AM, Abrahamsson P, N⊘rgaard AW. Development and evaluation of a liquid chromatography tandem mass spectrometry method for simultaneous determination of salivary melatonin, cortisol and testosterone. J Chromatogr B Analyt Technol Biomed Life Sci. 2011; 879:2527–32.

13. Jensen MA, Mortier L, Koh E, Keevil B, Hyttinen S, Hansen ÅM. An interlaboratory comparison between similar methods for determination of melatonin, cortisol and testosterone in saliva. Scand J Clin Lab Invest. 2014; 74:454–61.


14. CLSI. Liquid chromatography-mass spectrometry methods. 1st ed. CLSI C62-A. Wayne, PA: Clinical and Laboratory Standards Institute;2014.
15. Vogeser M, Kratzsch J, Ju Bae Y, Bruegel M, Ceglarek U, Fiers T, et al. Multicenter performance evaluation of a second generation cortisol assay. Clin Chem Lab Med. 2017; 55:826–35.


16. Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001; 26:165–73.


17. Nölte I, Lütkhoff AT, Stuck BA, Lemmer B, Schredl M, Findeisen P, et al. Pineal volume and circadian melatonin profile in healthy volunteers: an interdisciplinary approach. J Magn Reson Imaging. 2009; 30:499–505.


Fig. 1
Comparison plots between LC-MS/MS and immunoassays. Top: (A) Passing-Bablok regression and (B) Bland-Altman plot to compare salivary melatonin concentrations measured by LC-MS/MS assay and ELISA. Bottom: (C) Passing-Bablok regression and (D) Bland-Altman plot to compare salivary cortisol concentrations measured by LC-MS/MS assay and ECLIA.
Abbreviations: LC-MS/MS, liquid chromatography-tandem mass spectrometry; ECLIA, electrochemiluminescence immunoassay.
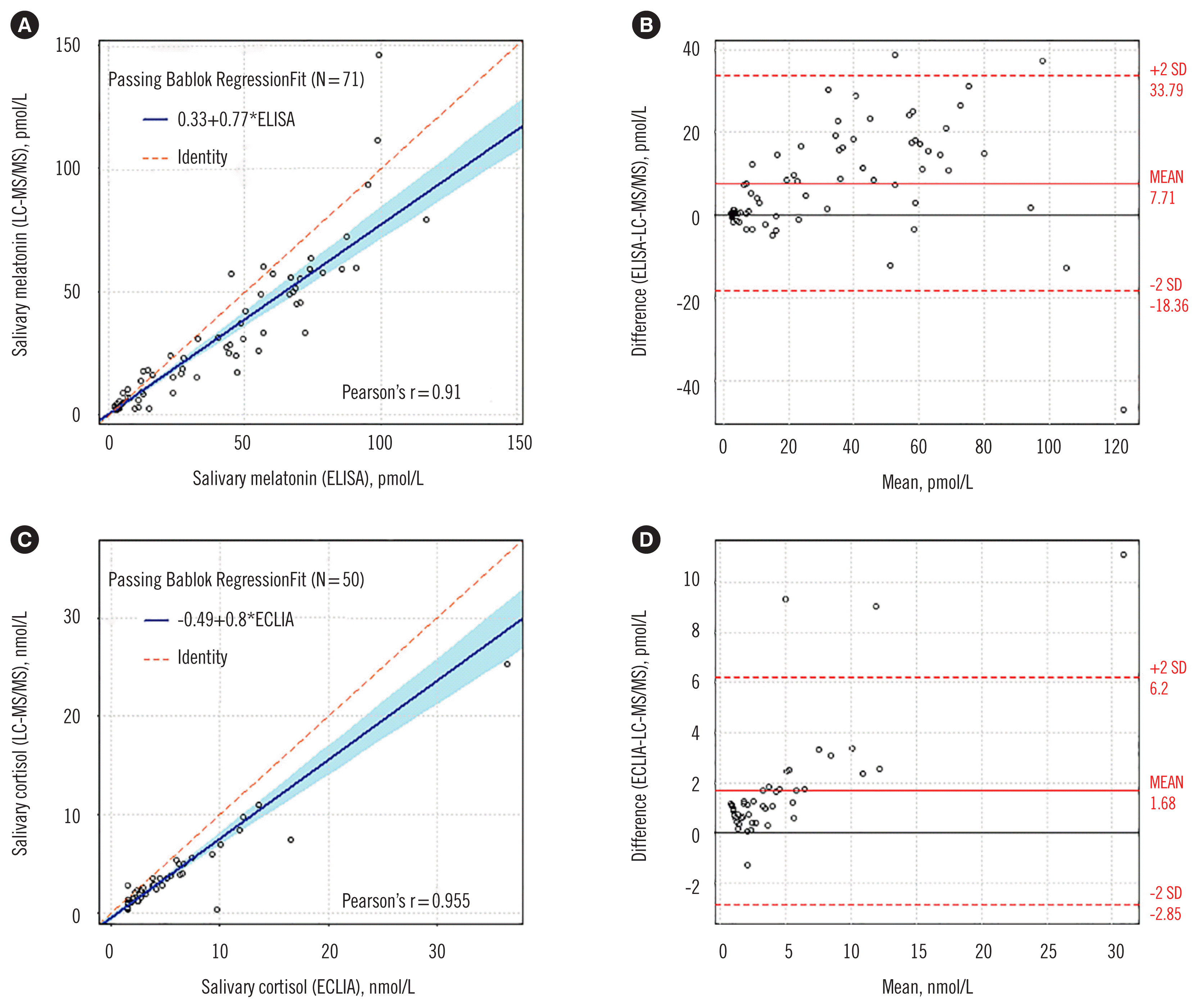
Fig. 2
Melatonin and cortisol profiles of two participants measured by LC-MS/MS assay and immunoassays.
Abbreviations: ECLIA, electrochemiluminescence immunoassay; LC-MS/MS, liquid chromatography-tandem mass spectrometry.
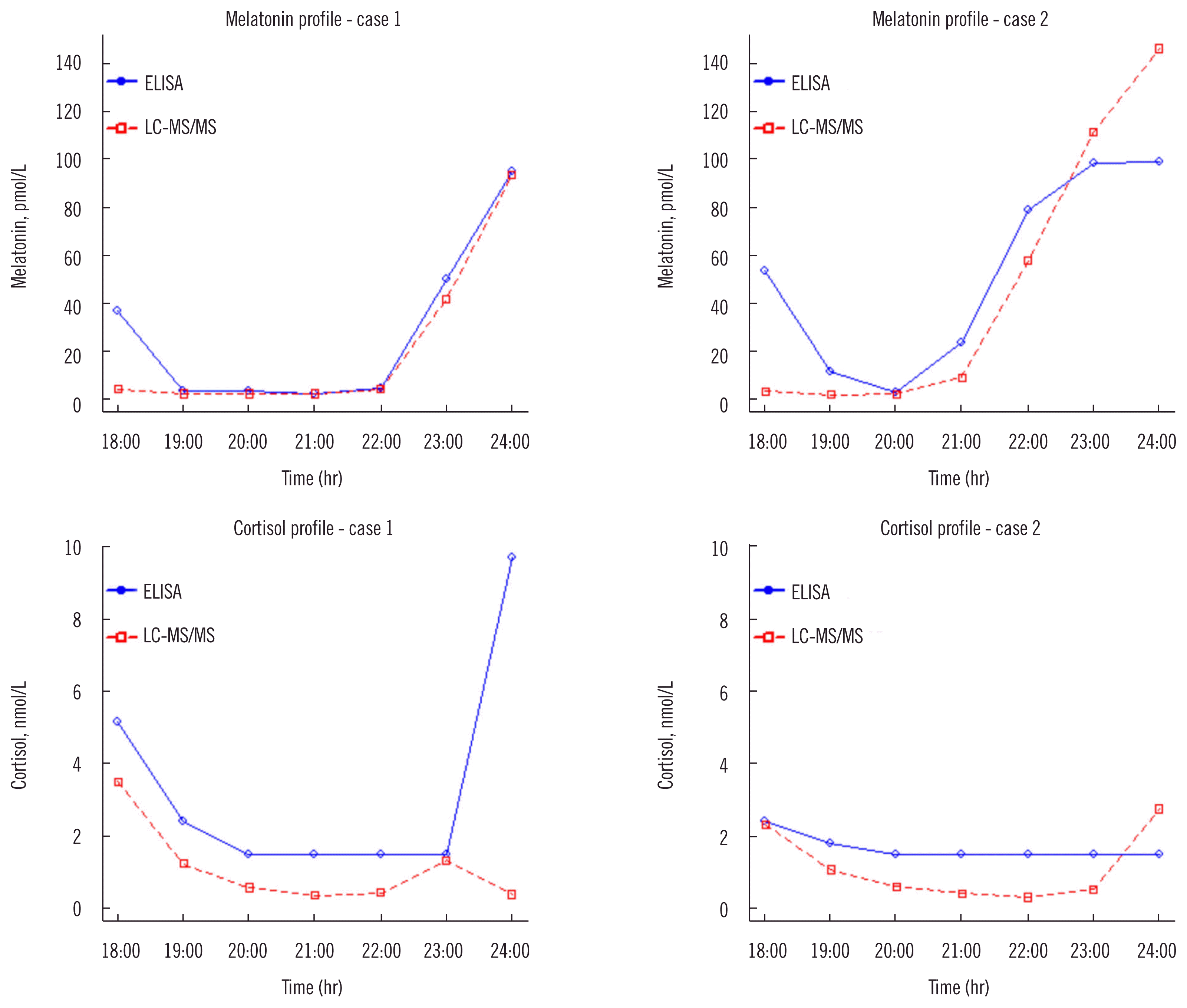
Table 1
The performance of LC-MS/MS assay to measure melatonin and cortisol concentrations
| Analyte | Assay range | r | LOD | LLOQ* | Accuracy (%) | Precision (CV, %)† | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Intra-assay (N=5) | Inter-assay (N=30) | ||||||||||
|
|
|
||||||||||
| 8.6 (0.55) | 86 (5.52) | 344 (22.07) | 8.6 (0.55) | 86 (5.52) | 344 (22.07) | ||||||
| Melatonin | 2.15–430 pmol/L | 0.997 | 0.43 pmol/L | 2.15 pmol/L | 100.3–102.2 | 3.6 | 3.3 | 4.9 | 6.8 | 5.4 | 3.5 |
|
|
|||||||||||
| Cortisol | 0.14–27.59 nmol/L | 0.999 | 0.03 nmol/L | 0.14 nmol/L | 96.9–107.8 | 2.6 | 3.1 | 3.1 | 4.7 | 4.7 | 3.7 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download