INTRODUCTION
MATERIALS AND METHODS
Sample details
First measurement
Storage temperature
Storage time and second measurement
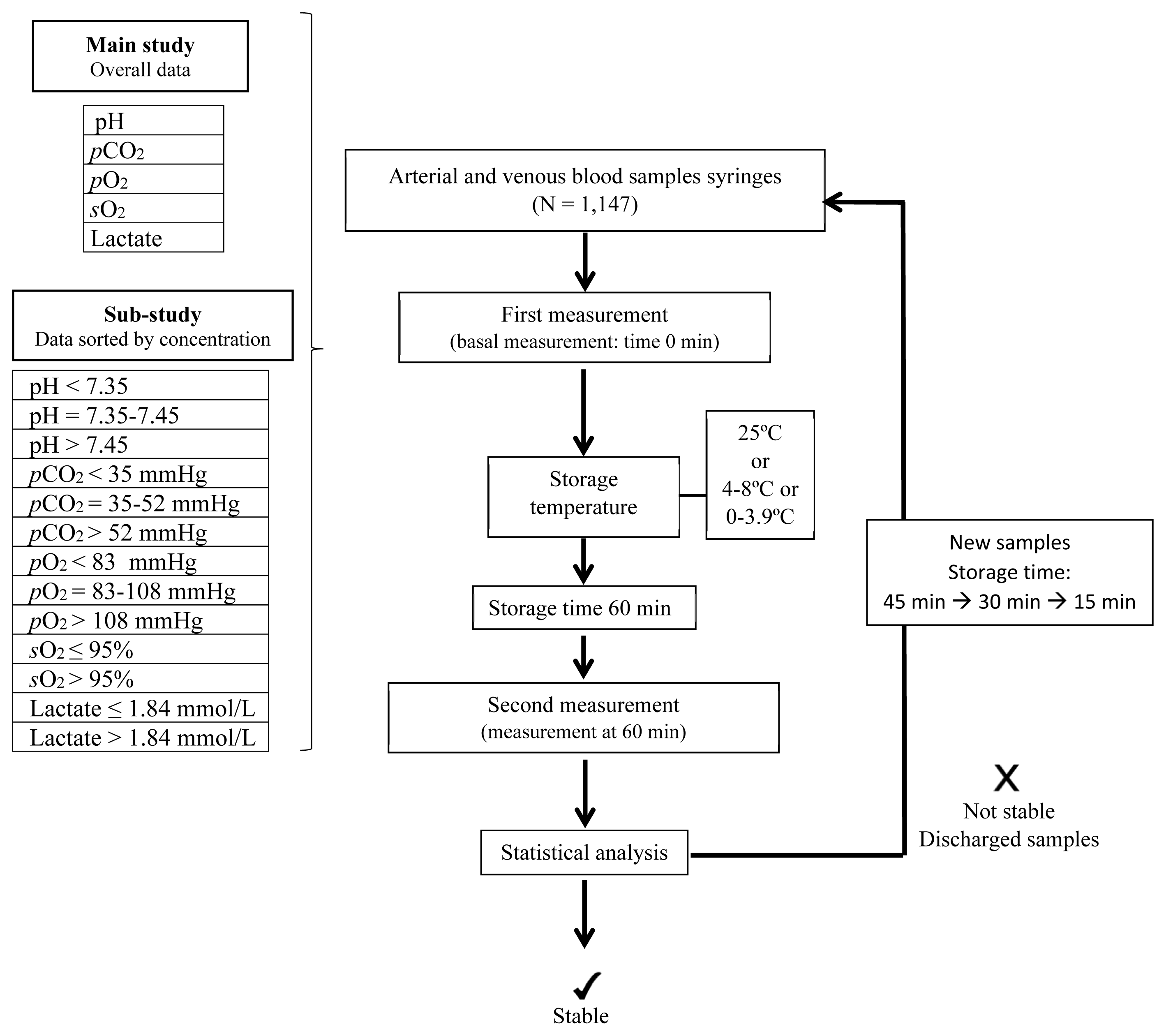 | Fig. 1Study flow diagram. The stability study began with storing the samples for 60 minutes. If any parameter was not stable for ≤60 minutes, the study was started again with new samples for 45 minutes. If stability was still an issue, the experiment was carried out for 30 and 15 minutes, sequentially. Independent sample groups were used each time. Measurements were conducted using an ABL800 analyzer (Radiometer, Copenhagen, Denmark).
Abbreviations: pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; sO2, oxygen saturation.
|
Main study and substudy
Statistical analysis
RESULTS
Within-run imprecision study
Table 1
Main study
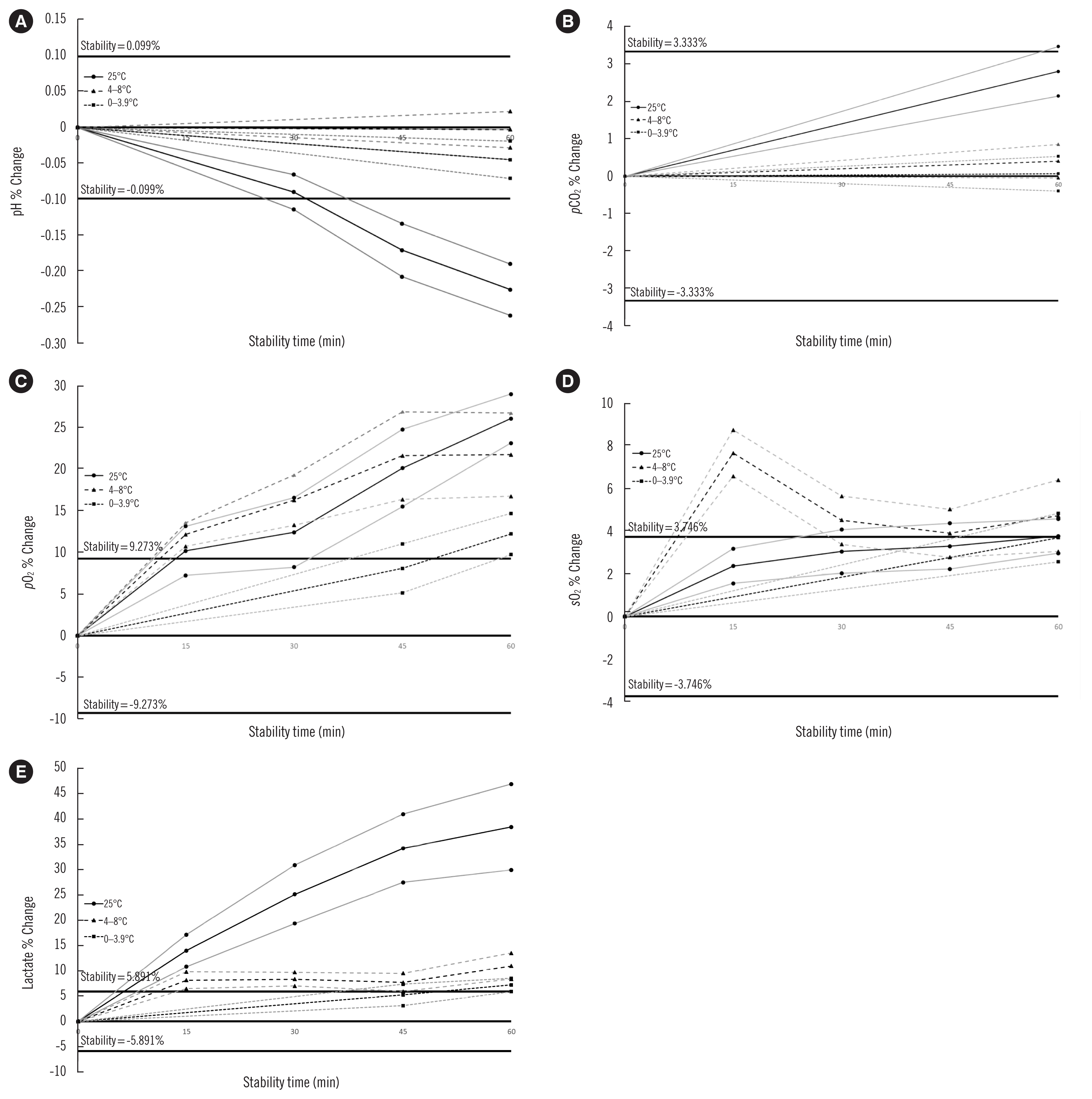 | Fig. 2Mean bias from baseline for pH (A), pCO2 (B), pO2 (C), sO2 (D), and lactate concentration (E) at 25°C (solid black curve), 4–8°C (long dashed black curve), and 0–3.9°C (short dashed black curve). The two adjacent grey curves on either side represent the 95% confidence interval of the mean curve. The solid black lines correspond to stability (%) calculated as a CV percentage overall (within-run imprecision)×1.65. Data are displayed in Table 2. Independent sample groups were used for each time, even though there is a connection between lines.
Abbreviations: pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; sO2, oxygen saturation; CV, coefficient of variation.
|
Table 2
| N | Temperature (°C) | Time (min) | PD (95% CI) (%)* | S (%)† | |
|---|---|---|---|---|---|
| pH | 92 | 25 | 30 | −0.090 (−0.114; −0.066) | 0.099 |
| 97 | 4–8 | ≤60 | −0.003 (−0.028; 0.022) | ||
| 89 | 0–3.9 | ≤60 | −0.045 (−0.071; −0.019) | ||
|
|
|||||
| pCO2 | 90 | 25 | ≤60 | 2.81 (2.15; 3.47) | 3.33 |
| 110 | 4–8 | ≤60 | 0.405 (−0.043; 0.853) | ||
| 90 | 0–3.9 | ≤60 | 0.070 (−0.395; 0.535) | ||
|
|
|||||
| pO2 | 84 | 25 | <15 | 10.2 (7.25; 13.1) | 9.27 |
| 190 | 4–8 | <15 | 12.2 (10.7; 13.6) | ||
| 66 | 0–3.9 | 45 | 8.09 (5.15; 11.0) | ||
|
|
|||||
| sO2 | 66 | 25 | 15 | 2.37 (1.56; 3.18) | 3.75 |
| 104 | 4–8 | <15 | 7.68 (6.59; 8.76) | ||
| 81 | 0–3.9 | ≤60 | 3.71 (2.57; 4.84) | ||
|
|
|||||
| Lactate | 65 | 25 | <15 | 14.0 (10.8; 17.1) | 5.89 |
| 39 | 4–8 | <15 | 8.13 (6.46; 9.81) | ||
| 27 | 0–3.9 | 45 | 5.22 (3.09; 7.34) | ||
PD in bold indicates significant stability time (PD<S). Groups that were stable for the entire study period are identified as ≤60, while those that were not stable for even 15 min are designated as <15.
* PD (%) calculated as ∑ (Yi−Xi)/Xi)/n×100. The first (Xi) and the second (Yi) sample result for each parameter and storage condition;
Substudy
Table 3
| Temperature (°C) | N | Time (min) | PD (%)* | S (%)† | N | Time (min) | PD (%)* | S (%)† | N | Time (min) | PD (%)* | S (%)† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH<7.35 | pH=7.35–7.45 | pH>7.45 | ||||||||||
| 25 | 30 | 30 | 0.088 | 0.116 | 29 | 15 | 0.038 | 0.066 | 29 | 15 | 0.020 | 0.099 |
| 4–8 | 30 | ≤60 | 0.023 | 36 | ≤60 | 0.032 | 31 | ≤60 | 0.051 | |||
| 0–3.9 | 29 | ≤60 | 0.088 | 31 | ≤60 | 0.015 | 29 | ≤60 | 0.024 | |||
|
|
||||||||||||
| pCO2<35 mmHg | pCO2=35–52 mmHg | pCO2>52 mmHg | ||||||||||
| 25 | 25 | 30 | 0.304 | 2.77 | 32 | 45 | 0.021 | 2.72 | 27 | ≤60 | 0.618 | 3.42 |
| 4–8 | 31 | ≤60 | 0.203 | 55 | ≤60 | 0.605 | 24 | ≤60 | 0.206 | |||
| 0–3.9 | 33 | ≤60 | 0.193 | 31 | ≤60 | 0.520 | 26 | ≤60 | 0.612 | |||
|
|
||||||||||||
| pO2<83 mmHg | pO2=83–108 mmHg | pO2>108 mmHg | ||||||||||
| 25 | 34 | 15 | 8.68 | 8.83 | 26 | <15 | 17.8 | 10.5 | 27 | 30 | 3.93 | 7.21 |
| 4–8 | 116 | <15 | 10.6 | 31 | <15 | 17.2 | 43 | <15 | 12.8 | |||
| 0–3.9 | 53 | ≤60 | 6.99 | 30 | <15 | 18.7 | 34 | <15 | 16.6 | |||
|
|
||||||||||||
| sO2≤95 % | sO2>95 % | |||||||||||
| 25 | 33 | 15 | 4.38 | 6.40 | 33 | ≤60 | 0.379 | 4.49 | ||||
| 4–8 | 104 | <15 | 7.68 | 33 | ≤60 | 0.566 | ||||||
| 0–3.9 | 49 | ≤60 | 5.56 | 32 | ≤60 | 1.87 | ||||||
|
|
||||||||||||
| Lactate≤1.84 mmol/L | Lactate>1.84 mmol/L | |||||||||||
| 25 | 36 | <15 | 18.6 | 9.49 | 29 | <15 | 8.23 | 4.69 | ||||
| 4–8 | 25 | <15 | 10.5 | 14 | 15 | 3.89 | ||||||
| 0–3.9 | 44 | ≤60 | 8.03 | 29 | ≤60 | 2.34 | ||||||
Groups that were stable for the entire study period are identified as ≤60, while those that were not stable for even 15 minutes are designated as <15. PD in bold indicates significant stability time (PD<S).
* PD (%) calculated as ∑ (Yi−Xi)/Xi)/n×100. The first (Xi) and the second (Yi) sample result for each parameter and storage condition;




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download