Dear Editor:
Secondary hematologic malignancy associated with chemotherapy or radiotherapy is common and mostly involves AML or MDS. However, therapy-related CML (tr-CML) is relatively rare as the progenitor cells in CML are less susceptible to DNA damage owing to their quiescent characteristics [
1,
2] and the oncogene is relatively unified to Philadelphia chromosome (Ph), which harbors the
BCR-ABL1 gene [
3]. A previous study on tr-CML revealed that the average time for CML development following the first course of therapy is 48 months (range: 23 to 192 months) [
2]. tr-CML has been shown to develop following radiotherapy and/or chemotherapy wherein alkylating agents or topoisomerase inhibitors are mostly used [
2,
4]. tr-CML has been previously reported in solid tumors, lymphoma, multiple myeloma, etc. [
2–
5]. Among them, Hodgkin’s lymphoma (HL) was the most common primary malignancy followed by CLL [
2]. Uterine, cervical, ovarian, and breast cancers were also common primary malignancies in tr-CML [
4]. The major
BCR-ABL1 is a common fusion gene in tr-CML, and
JAK2-BCR has been reported occasionally [
3,
5]. The clinical course of tr-CML seems similar to that of
de novo CML [
4]. Most patients presented clinical features of CML such as leukocytosis or anemia [
2–
5].
A few cases of preleukemic CML (termed “preclinical,” “asymptomatic,” or “smoldering” CML), which is characterized by the presence of
BCR-ABL1 without clinical features of CML, have been reported. However, most cases were not associated with cytotoxic therapy [
6–
8]. To the best of our knowledge, this is the first case of preleukemic CML related to cytotoxic therapy (therapy-related preleukemic CML) in Korea. Until now two cases of therapy-related preleukemic CML have been reported worldwide (
Table 1) [
9,
10]. Our case is unique because we confirmed normal karyotype before chemotherapy, suggesting that the therapy induced preleukemic CML. This study was approved by the Institutional Review Board of Kosin University Gospel Hospital, Busan, Korea (KUGH 2019-12-007) that waived informed consent from the patient.
Table 1
Patients developing therapy-related preleukemic CML
|
Patient |
Reference |
ID state |
preleukemic CML state |
|
|
|
Age (yr) |
Sex |
ID |
BM cytogenetics |
Type of therapy |
Interval ID-preleukemic CML |
Patient’s status |
BM cytogenetics |
Follow-up |
|
1 |
Berman et al. [9] |
82 |
M |
CLL |
NT |
CTx |
7 years |
Newly diagnosed MCL |
47,XY,t(9;22)(q34;q11.2),+mar[4]/46,XY[16] |
Developed clinical CML 8 months later |
|
|
2 |
Bolaños-Meade et al. [10] |
35 |
M |
HL |
NT |
CTx + RTx |
14 months |
Preparing autologous SCT |
46,XY,t(9;22)(q34;q11)[1]/46,XY[6] |
Developed clinical CML 16 months later |
|
|
3 |
Present case |
37 |
M |
T-LBL |
46,XY[20] |
CTx |
32 months |
Relapsed T-LBL |
46,XY,t(9;22)(q34;q11.2)[15]/46,XY[5] |
Regular follow-up proceeding for 8 months |

A 37-year-old man visited the Kosin university Gospel hospital presenting with a mass in his left oropharynx in November 2016 and was diagnosed as having T-lymphoblastic lymphoma (LBL). He showed normal karyotype, 46,XY [20], in his bone marrow with no evidence of lymphoma involvement. He underwent a left tonsillectomy and four cycles of systemic chemotherapy. The chemotherapy regimens included: one cycle of methotrexate, cytarabine, and dexamethasone; two cycles of cyclophosphamide, vincristine, doxorubicin, and dexamethasone; one cycle of methotrexate and cytarabine; and a final cycle of etoposide, methylprednisolone, cytarabine, and cisplatin. He achieved complete remission in December 2016 and subsequently underwent autologous stem cell transplantation in May 2017 after conditioning with busulfan, etoposide, and cyclophosphamide.
The lymphoma relapsed in his right tonsil in July 2019. The complete blood counts were as follows: Hb 141 g/L, platelets 240×109/L, and leukocytes 6.28×109/L with 34% segmented neutrophils, 1% band neutrophils, 53% lymphocytes, 5% monocytes, 5% eosinophils, and 2% basophils. Bone marrow examination showed no evidence of CML or lymphoma (the myeloid:erythroid ratio was 3.4:1).
The differential counts of the bone marrow aspirate were as follows: myeloblasts, 1.8%; promyelocytes, 1.8%; myelocytes, 14.3%; metamyelocytes, 15.4%; band neutrophils, 15.0%; segmented neutrophils, 16.1%; eosinophils, 2.5%; basophils, 0.5%; proerythroblasts, 0.5%; basophilic erythroblasts, 0.9%; polychromatic erythroblasts, 10.2%; orthochromatic erythroblasts, 8.2%; lymphocytes, 8.6%; monocytes, 1.6; and histiocytes, 2.5%. However, a 46,XY,t(9;22)(q34;q11.2)[15]/46,XY[
5] karyotype was detected (
Fig. 1A). Additionally, FISH analysis using the locus-specific identifier
BCR/
ABL (
ABL1) translocation, dual fusion probe (Cytocell, Cambridge, UK) revealed the
BCR and
ABL1 gene fusion in 76.5% of interphases (
Fig. 1B). Real-time PCR (Qiagen, Marseille, France) analysis revealed a normalized copy number of 29.5 for minor
BCR-ABL1 (e1a2). An additional real-time PCR for relapsed lymphoma was considered; however, the residual tonsillar biopsy sample was insufficient. Moreover, we attempted to identify
BCR-ABL1 retrospectively from the initial bone marrow sample and lymphoma tissue which were obtained in 2016; however, this proved unfeasible. The patient was diagnosed as having recurrent T-LBL and preleukemic CML. He was treated for relapsed lymphoma; regular examination for preleukemic CML was scheduled without tyrosine kinase inhibitor therapy.
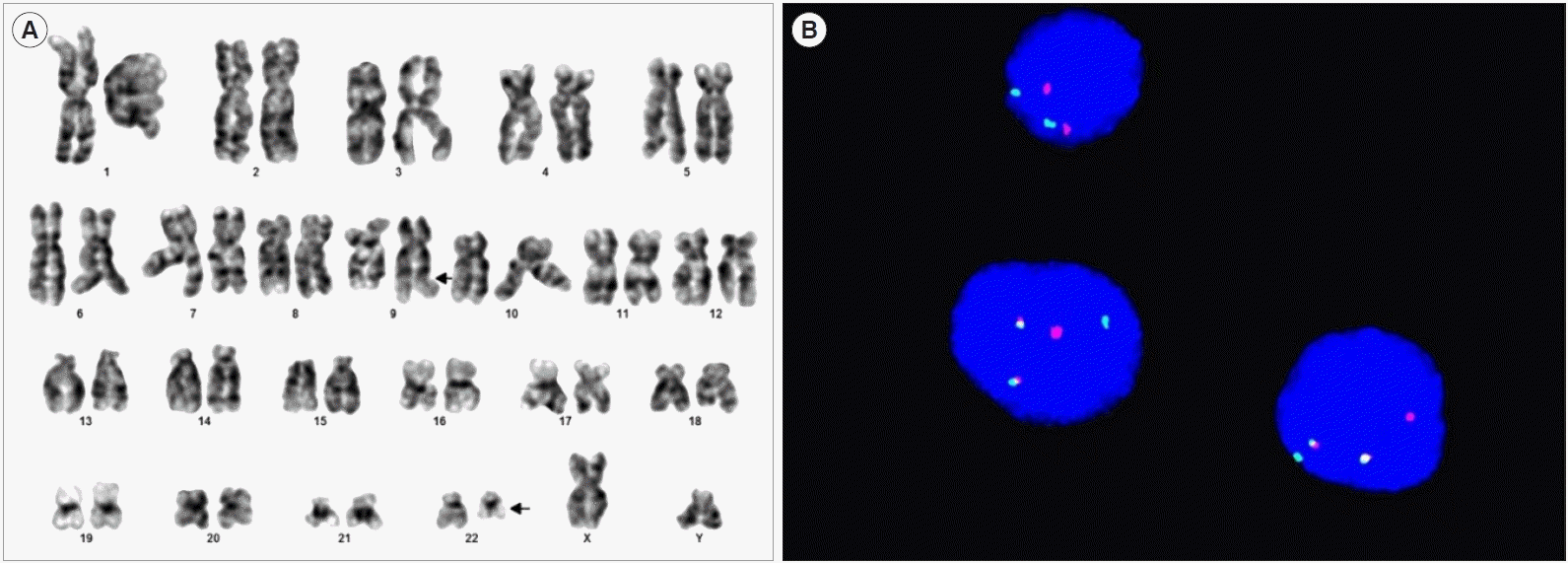 | Fig. 1Patient’s clinical features. (A) The karyotype analysis showed 46,XY,t(9;22)(q34;q11.2) in 15 of 20 cells evaluated (arrow). (B) The FISH study revealed fusion of ABL1 and BCR genes in 76.5% of interphase. 
|
To date, only two cases of therapy-related preleukemic CML have been reported (
Table 1) [
9,
10]. In one case, a 32-year-old man with HL received radiotherapy and chemotherapy. After 22 months, the presence of
BCR-ABL1 was detected and the patient was diagnosed as having preleukemic CML that progressed to clinical CML 16 months later [
9]. The other case involved an 82-year-old man diagnosed as having mantle cell lymphoma (MCL). He had a medical history of CLL, which had been treated with chemotherapy seven years previously and did not exhibit any clinical features; however,
BCR-ABL1 was detected when he was diagnosed as having MCL. He was diagnosed as having preleukemic CML, which developed into clinical CML within eight months [
10]. However, in both cases, the presence of the
BCR-ABL1 fusion gene was not evaluated before initial therapy. In contrast, in our study, chemotherapy-induced preleukemic CML was supported by the presence of normal chromosomes in the patient’s bone marrow karyotype before chemotherapy. There is no consensus on the standard treatment for preleukemic CML. However preemptive tyrosine kinase inhibitor (TKI) therapy is often considered and may delay the transformation to clinical CML [
6–
8].
This report suggests that BCR-ABL1 clones can be asymptomatic after cytotoxic therapy and require some time before progression to clinical CML. During preleukemic CML period TKI therapy can be used preemptively. We believe that this is the first reported case of preleukemic CML that occurred post cytotoxic therapy for T-LBL.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download