This article has been
cited by other articles in ScienceCentral.
Dear Editor,
Gout is a systemic disease characterized by recurrent, painful arthritis caused by the deposition of uric acid crystals [
1]. Uric acid crystal deposition occurs in tissues when serum uric acid level rises above the normal threshold. The pathological threshold of uric acid for hyperuricemia is 0.40 mmol/L [
2]. A deposit of uric acid crystals in patients with longstanding hyperuricemia is termed a tophus [
3]. Gouty tophi generally occur at the first metatarsophalangeal joints, ears, and finger pulps; however, although rare, gouty tophi have also been reported at non-skeletal sites including the bronchi, mitral valve, liver, breasts, and even bone marrow (BM) [
3–
5]. To date, only two cases of gout involving the BM have been reported [
4,
5], and neither of them was accompanied by other hematologic malignancies such as plasma cell myeloma (PCM). Therefore, we report the first case of gouty tophi in the BM of a patient with PCM. This study was approved by the Institutional Review Board of Soonchunhyang University Hospital, Seoul, Korea (IRB File No. 2019-04-002).
A 67-year-old woman, who had been receiving dialysis for four yrs owing to monoclonal gammopathy of undetermined significance and end-stage renal disease, was admitted to the Soonchunhyang University Hospital, Seoul, for an evaluation of frequent catheter occlusion. She also complained of severe pain in the distal interphalangeal joints in both hands. The initial laboratory findings were as follows: hemoglobin, 94 g/L (reference range, 120–160 g/L); white blood cell count, 7.1×109/L (4.0–10.0×109/L); platelet count, 146×109/L (130–450×109/L); prothrombin time (PT), 9.9 seconds (9.3–11.6 seconds); activated partial thromboplastin time (aPTT), 32.0 seconds (27.8–41.7 seconds); fibrinogen, 2.95 g/L (2.09–3.77 g/L); D-dimer, 9,812 μg/L (0–243 μg/L); anti-thrombin, 26% (65–129%); protein C, 59% (70–148%); free protein S, 58.8% (69.4–138.3%); blood urea nitrogen (BUN), 17.11 mmol/L (2.14–7.14 mmol/L); serum creatinine, 431.40 μmol/L (44.20–106.08 μmol/L); uric acid, 0.30 mmol/L (0.14–0.42 mmol/L); total protein, 50 g/L (64–83 g/L); albumin, 16 g/L (35–52 g/L); IgG, 8.96 g/L (7.00–16.00 g/L); IgA, 1.81 g/L (0.70–4.00 g/L); IgM, 0.88 g/L (0.40–2.30 g/L); free light kappa chain, 0.13 g/L (0.03–0.19 g/L); free light lambda chain, 10.39 g/L (0.05–0.26 g/L); free light kappa chain/free light lambda chain ratio, 0.12 (0.26–1.65); and β2-microglobulin, 2,412.67 nmol/L (84.82–203.56 nmol/L).
Serum protein electrophoresis revealed two monoclonal peaks in the gamma region and immunofixation electrophoresis confirmed the presence of double gammopathy, IgG-lambda. The level of monoclonal protein was 6.6 g/L. Eight days after admission, the uric acid level increased to 0.52 mmol/L. A BM aspirate smear showed that plasma cells accounted for 45.2% of all nucleated cells, and a BM section revealed focally infiltrated plasma cells with multiple uric acid crystals (gouty tophi) with foreign body reaction (
Fig. 1). Immunophenotypic analysis revealed that the plasma cells were CD19−, CD56dim+, and CD45− and showed cytoplasmic lambda light chain restriction (tumor burden: 31.5%). No apparent cytogenetic abnormalities were found by FISH for t(4;14)(p16;q32), t(14;16)(q32;q23), t(11;14)(q13;q32), 17p13 deletions, 1q gains, and chromosome 13 abnormalities and the chromosomal analysis was also normal. The patient was diagnosed as having symptomatic PCM with gout and was started on a treatment regime of bortezomib, melphalan, and prednisone. However, 10 days after chemotherapy was initiated, the patient died of uncontrolled acute gastrointestinal bleeding induced by disseminated intravascular coagulation (DIC). Clinical manifestations of our case along with those of the previously reported cases are summarized in
Table 1.
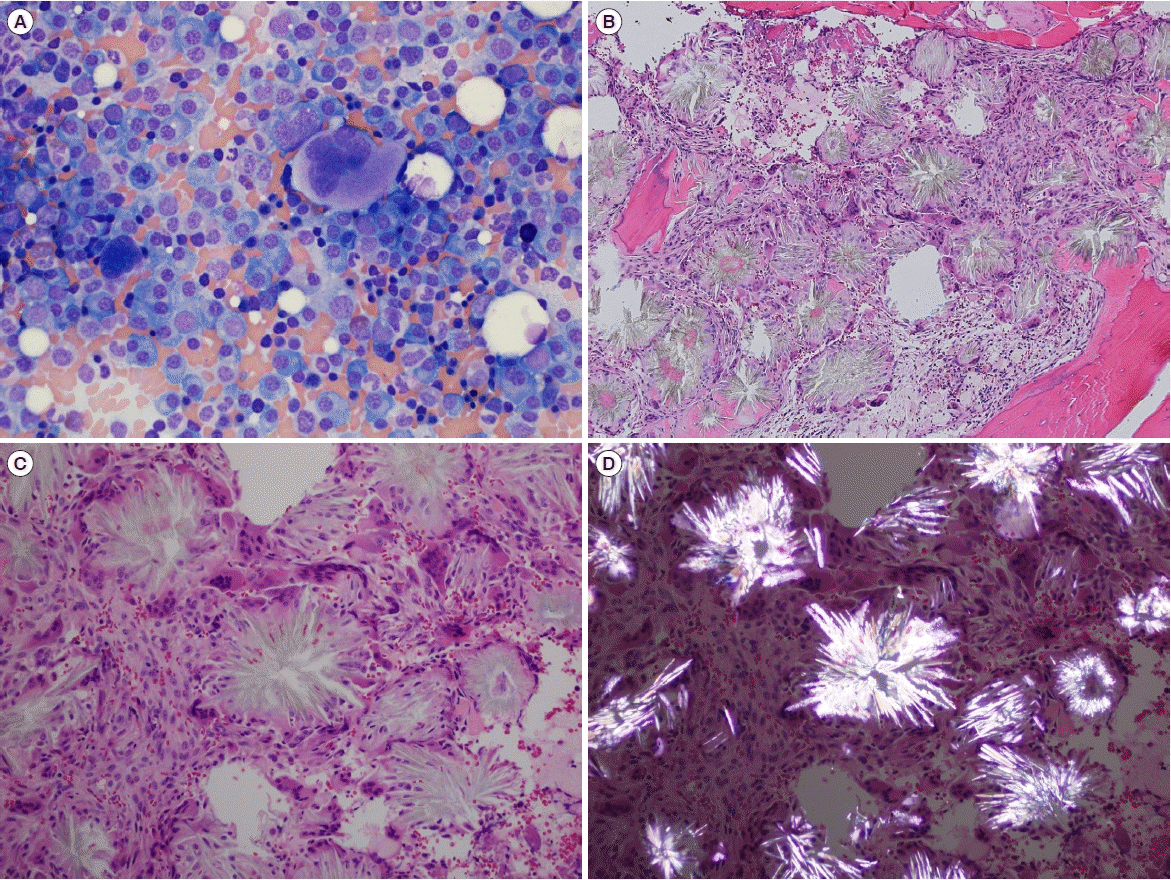 | Fig. 1Bone marrow (BM) aspirate smear and biopsy findings. (A) Plasma cells on the BM aspirate smear (Wright-Giemsa stain, ×400). (B, C) Fine, needle-shaped, radially oriented crystals surrounded by foreign-body type giant cells on the BM section (hematoxylin and eosin stain, ×100 and ×200). (D) Shiny, strong negatively birefringent crystals against a dark background observed by birefringence microscopy, consistent with uric acid crystals (hematoxylin and eosin stain, ×200). 
|
Table 1
Clinical manifestations of reported BM gouty tophi
|
Published cases |
Underlying disease |
Present illness |
Reason for BM study |
BM Diagnosis |
Treatment |
Disease course |
|
Choi et al. [4] |
HTN, ESRD, hypothyroidism |
Pain in nodules and plaques on extremities |
Anemia, Neutropenia |
BM gouty tophi only |
Allopurinol, Colchicine, Prednisolone |
Renal: aggravated Skin: stationary |
|
Agarwaal et al. [5] |
Gout, Hepatitis C infection |
NI |
Leukopenia, Thrombocytopenia |
BM gouty tophi only |
NI |
NI |
|
Present case |
MGUS, ESRD |
Catheter occlusion, Pain in hand joints |
Anemia, For evaluation of MGUS |
Plasma cell myeloma with BM gouty tophi |
Bortezomib, Melphalan, Prednisone |
Died after 1st CTx |

Hyperuricemia can occur owing to overproduction of uric acid through liver metabolism and cell turnover or underexcretion by the kidneys and gut [
1,
2]. Although hyperuricemia is a classic feature of gout, blood uric acid levels can be normal during an attack [
6], as in our case. In general, gouty tophi have a predilection for the distal parts of the body because of distal circulatory stasis [
7]. Increased plasma cells near the BM vascular niche in myeloma patients might cause circulatory stasis and uric acid deposition [
8]. In addition, a tophus is a chronic inflammatory granulomatous response to uric acid crystals. Monocytes engulfing uric acid crystals can release proinflammatory cytokines, such as interleukin 1β and tumor necrosis factor α, and induced neutrophils can also play a role in tophus formation [
1–
3]. These inflammatory reactions in patients with gout can also increase thrombotic tendencies [
8,
9], which might have led to the DIC state in our patient.
We believe that PCM patients with gouty tophi in the BM should be better cared for because of the increased possibility of thrombotic tendency during treatment. Further clinical studies are needed to optimize treatment strategies for the patients with clinical process like our case.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download