INTRODUCTION
MATERIALS AND METHODS
Recipient population
Measurement of anti-AT1Rs using enzyme-linked immunosorbent assay (ELISA)
Detection of AECAs using flow cytometric endothelial cell crossmatch (ECXM) assay
HLA testing
Kidney histology
Data collection and statistical analysis
RESULTS
Characteristics of the study population
Table 1
| Characteristics | Recipients (N=94)* |
|---|---|
| Age (yr) | 49.5 (39–56) |
|
|
|
| Gender, male | 57 (60.6%) |
|
|
|
| BMI | 23.1±3.8 |
|
|
|
| Diagnosis | |
| Diabetic nephropathy | 25 (29.8%) |
| IgA nephropathy | 16 (17.0%) |
| Hypertensive nephrosclerosis | 15 (16.0%) |
| Glomerulonephritis | 10 (10.6%) |
| Other causes | 7 (7.4%) |
| Unknown | 18 (19.2%) |
|
|
|
| Re-transplantation | 1 (1.1%) |
|
|
|
| Induction therapy regimen | |
| Anti-thymocyte globulin | 10 (10.6%) |
| Basiliximab | 84 (89.4%) |
|
|
|
| Maintenance regimen immunosuppressants | |
| CsA+MMF+PD | 13 (13.8%) |
| FK+MMF+PD | 79 (84.0%) |
| Sirolimus or everolimus combination | 2 (2.1%) |
|
|
|
| HLA mismatches | |
| Class 1 (HLA-A, -B) | 2 (1–3) |
| Class 2 (HLA-DR) | 1 (0–1) |
|
|
|
| cPRA | |
| 0% | 72 (76.6%) |
| <50% | 18 (19.1%) |
| ≥50% | 4 (4.3%) |
|
|
|
| Pre-transplant AECA (+) | 22 (23.4%) |
|
|
|
| Pre-transplant anti-AT1R levels, U/mL | 10.2±4.8 |
|
|
|
| AR, during F/U period | 43 (45.7%) |
|
|
|
| MVI only (g+ptc≥2), during F/U period | 6 (6.4%) |
* Continuous variables are reported as mean±SD or median (interquartile range) and categorical variables are listed as total number (%). Kolmogorov-Smirnov test was employed for testing normality assumption.
Abbreviations: LDKT, living donor kidney transplantation; BMI, body mass index; CsA, cyclosporine A; MMF, mycophenolate mofetil; PD, prednisolone; FK, tacrolimus; cPRA, calculated panel reactive antibodies; AECA, anti-endothelial cell antibodies; Anti-AT1R, anti- angiotensin II type 1 receptor antibodies; MVI, microvascular inflammation; g, glomerulitis; ptc, peritubular capillaritis; AR, acute rejection; F/U, follow-up.
Correlation between clinical outcomes and anti-AT1R levels and AECA results
Table 2
| Anti-AT1R-negative (<10 U/mL) (N=45)* | Anti-AT1R at-risk (10–17 U/mL) (N=41)* | Anti-AT1R-positive (>17 U/mL) (N=8)* | P | AECA (−) (N=72)* | AECA (+) (N=22)* | P | |
|---|---|---|---|---|---|---|---|
| Age (yr) | 50 (40–55) | 50 (39–56) | 45.5 (39.5–59.5) | 0.975 | 50 (40–56) | 46 (37–53) | 0.437 |
|
|
|||||||
| Gender, male | 32 (71.1%) | 22 (53.7%) | 3 (37.5%) | 0.097 | 43 (59.7%) | 14 (63.6%) | 0.807 |
|
|
|||||||
| BMI | 23.6±3.5 | 22.9±4.2 | 20.7±2.4 | 0.069 | 23.5±4.0 | 21.6±2.9 | 0.05 |
|
|
|||||||
| HLA mismatches | |||||||
| Class 1 (HLA-A, -B) | 2 (1–3) | 2 (2–3) | 2 (1–2.5) | 0.683 | 2 (1–3) | 2 (2–3) | 0.712 |
| Class 2 (HLA-DR) | 1 (1-1) | 1 (0–2) | 1 (0.5–1) | 0.749 | 1 (1-1) | 1 (0–2) | 0.683 |
|
|
|||||||
| cPRA | 0.024 | 0.72 | |||||
| 0% | 39 (86.7%) | 29 (70.7%) | 4 (50%) | 54 (75.0%) | 18 (81.8%) | ||
| <50% | 6 (13.3%) | 8 (19.5%) | 4 (50%) | 14 (19.4%) | 4 (18.2%) | ||
| ≥50% | 0 (0%) | 4 (9.8%) | 0 (0%) | 4 (5.6%) | 0 (0%) | ||
|
|
|||||||
| Induction therapy regimen | 1.0 | 1.0 | |||||
| Anti-thymocyte globulin | 5 (11.1%) | 4 (9.8%) | 1 (12.5%) | 8 (11.1%) | 2 (9.1%) | ||
| Basiliximab | 40 (88.9%) | 37 (90.2%) | 7 (87.5%) | 64 (88.9%) | 20 (90.9%) | ||
|
|
|||||||
| Maintenance regimen immunosuppressants | 0.118 | 0.84 | |||||
| CsA+MMF+PD | 10 (22.2%) | 2 (4.9%) | 1 (12.5%) | 11 (15.3%) | 2 (9.1%) | ||
| FK+MMF+PD | 34 (75.6%) | 38 (92.7%) | 7 (87.5%) | 59 (81.9%) | 20 (90.9%) | ||
| Sirolimus or everolimus combination | 1 (2.2%) | 1 (2.4%) | 0 (0%) | 2 (2.8%) | 0 (0%) | ||
|
|
|||||||
| AR | |||||||
| Within 1 month post-transplant | 8 (17.8%) | 10 (24.4%) | 0 (0%) | 0.468 | 13 (18.1%) | 5 (22.7%) | 0.627 |
| Within 3 months post-transplant | 8 (17.8%) | 13 (31.7%) | 1 (12.5%) | 0.245 | 16 (22.2%) | 6 (27.3%) | 0.625 |
| Within 6 months post-transplant | 9 (20.0%) | 16 (39.0%) | 2 (25.0%) | 0.153 | 19 (26.4%) | 8 (36.4%) | 0.368 |
| Within 12 months post-transplant | 10 (22.2%) | 17 (41.5%) | 2 (25.0%) | 0.152 | 21 (29.2%) | 8 (36.4%) | 0.523 |
| During F/U period | 17 (37.8%) | 23 (56.1%) | 3 (37.5%) | 0.139 | 29 (40.3%) | 14 (63.6%) | 0.062 |
|
|
|||||||
| AR or MVI only | |||||||
| Within 1 month post-transplant | 9 (20.0%) | 13 (31.7%) | 0 (0%) | 0.247 | 16 (22.2%) | 6 (27.3%) | 0.625 |
| Within 3 months post-transplant | 11 (24.4%) | 16 (39.0%) | 1 (12.5%) | 0.196 | 19 (26.4%) | 9 (40.9%) | 0.196 |
| Within 6 months post-transplant | 12 (26.7%) | 19 (46.3%) | 2 (25.0%) | 0.138 | 22 (30.6%) | 11 (50.0%) | 0.099 |
| Within 12 months post-transplant | 13 (28.9%) | 20 (48.8%) | 2 (25.0%) | 0.128 | 24 (33.3%) | 11 (50.0%) | 0.161 |
| During F/U period | 20 (44.4%) | 26 (63.4%) | 3 (37.5%) | 0.101 | 32 (44.4%) | 17 (77.3%) | 0.008 |
|
|
|||||||
| Pre-transplant anti-AT1R levels, U/mL | 6.26±2.2 | 12.50±1.6 | 20.72±3.1 | <0.001 | 10.0±4.8 | 11.0±5.1 | 0.412 |
|
|
|||||||
| Pre-transplant AECA (+) | 10 (22.2%) | 9 (22.0%) | 3 (37.5%) | 0.616 | |||
* Continuous variables are reported as mean±SD or median (interquartile range) and categorical variables are listed as total number (%). Kolmogorov-Smirnov test was employed for test of normality assumption.
Abbreviations: ECXM, endothelial cell crossmatch; Anti-AT1R, anti-angiotensin II type 1 receptor antibodies; AECA, anti-endothelial cell antibodies; BMI, body mass index; cPRA, calculated panel reactive antibodies; CsA, cyclosporine A; MMF, mycophenolate mofetil; PD, prednisolone; FK, tacrolimus; AR, acute rejection; MVI, microvascular inflammation.
Table 3
| Univariate analysis | Multivariate analysis* | Multivariate analysis† | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| AR within 6 months post-transplant | |||||||||
| HLA mismatches | |||||||||
| Class 1 (HLA-A, -B) | 1.47 | 0.98–2.20 | 0.060‡ | 1.44 | 0.84–2.46 | 0.181 | 1.36 | 0.83–2.25 | 0.225 |
| Class 2 (HLA-DR) | 2.50 | 1.23–5.02 | 0.010‡ | 2.02 | 0.86–4.75 | 0.106 | 1.89 | 0.84–4.24 | 0.124 |
| Pre-transplant anti-AT1R ≥11.5 U/mL | 2.74 | 1.09–6.86 | 0.032‡ | 4.11 | 1.44–11.79 | 0.009 | - | - | - |
| AECA (+) | 1.59 | 0.28–4.40 | 0.368 | - | - | - | 2.09 | 0.66–6.59 | 0.208 |
|
|
|||||||||
| AR within 12 months post-transplant | |||||||||
| HLA mismatches | |||||||||
| Class 1 (HLA-A, -B) | 1.50 | 1.01–2.23 | 0.046‡ | 1.52 | 0.92–2.52 | 0.103 | 1.44 | 0.89–2.33 | 0.134 |
| Class 2 (HLA-DR) | 2.14 | 1.11–4.16 | 0.024‡ | 1.59 | 0.72–3.51 | 0.251 | 1.54 | 0.72–3.32 | 0.267 |
| Pre-transplant anti-AT1R ≥11.5 U/mL | 2.25 | 0.92–5.49 | 0.077‡ | 3.11 | 1.15–8.43 | 0.026 | - | - | - |
| AECA (+) | 1.39 | 0.51–3.50 | 0.523 | - | - | - | 1.73 | 0.57–5.24 | 0.335 |
|
|
|||||||||
| AR during F/U period | |||||||||
| HLA mismatches | |||||||||
| Class 1 (HLA-A, -B) | 1.26 | 0.98–1.61 | 0.069‡ | 1.20 | 0.89–1.62 | 0.228 | 1.18 | 0.88–1.58 | 0.260 |
| Class 2 (HLA-DR) | 1.55 | 1.01–2.39 | 0.046‡ | 1.41 | 0.86–2.29 | 0.173 | 1.36 | 0.85–2.17 | 0.207 |
| Pre-transplant anti-AT1R ≥11.5 U/mL | 1.85 | 1.02–3.37 | 0.044‡ | 2.09 | 1.14–3.85 | 0.018 | - | - | - |
| AECA (+) | 1.84 | 0.97–3.48 | 0.062‡ | - | - | - | 1.92 | 1.01–3.66 | 0.046 |
|
|
|||||||||
| AR or MVI only during F/U period | |||||||||
| HLA mismatches | |||||||||
| Class 1 (HLA-A, -B) | 1.32 | 1.05–1.66 | 0.018‡ | 1.21 | 0.92–1.60 | 0.175 | 1.27 | 0.96–1.67 | 0.095 |
| Class 2 (HLA-DR) | 1.74 | 1.15–2.62 | 0.008‡ | 1.50 | 0.94–2.39 | 0.090 | 1.41 | 0.91–2.20 | 0.127 |
| Pre-transplant anti-AT1R ≥11.5 U/mL | 1.32 | 1.75–2.32 | 0.342 | 1.47 | 0.83–2.62 | 0.185 | - | - | - |
| AECA (+) | 2.23 | 1.23–4.02 | 0.008‡ | - | - | - | 2.47 | 1.35–4.53 | 0.004 |
Table 4
| Anti-AT1R <11.5 U/mL and AECA (−) (N=47)* | Anti-AT1R ≥11.5 U/mL and AECA (−) (N=25)* | Anti-AT1R <11.5 U/mL and AECA (+) (N=11)* | Anti-AT1R ≥11.5 U/mL and AECA (+) (N=11)* | P | |
|---|---|---|---|---|---|
| Age (yr) | 47.9±10.8 | 47.4±12.3 | 50.2±10.3 | 40.8±13.0 | 0.331 |
|
|
|||||
| Gender, male | 30 (63.8%) | 13 (52.0%) | 9 (81.8%) | 5 (45.5%) | 0.242 |
|
|
|||||
| BMI | 23.6±3.6 | 23.3±4.7 | 22.6±2.9 | 20.6±2.8 | 0.089 |
|
|
|||||
| HLA mismatches | |||||
| Class 1 (HLA-A,-B) | 2 (1–3) | 2 (1–3) | 3 (2–3) | 2 (1–2) | 0.666 |
| Class 2 (HLA-DR) | 1 (1-1) | 1 (0–1) | 1 (0–2) | 1 (0–2) | 0.822 |
|
|
|||||
| cPRA | 0.526 | ||||
| 0% | 37 (78.7%) | 17 (68.0%) | 10 (90.9%) | 8 (72.7%) | |
| <50% | 9 (19.2%) | 5 (20.0%) | 1 (9.1%) | 3 (27.3%) | |
| ≥50% | 1 (2.1%) | 3 (12.0%) | 0 (0%) | 0 (0%) | |
|
|
|||||
| Induction therapy regimen | 0.61 | ||||
| Anti-thymocyte globulin | 5 (10.6%) | 3 (12.0%) | 2 (18.2%) | 0 (0%) | |
| Basiliximab | 42 (89.4%) | 22 (88.0%) | 9 (81.8%) | 11 (100%) | |
|
|
|||||
| Maintenance regimen immunosuppressants | 0.704 | ||||
| CsA+MMF+PD | 8 (17.0%) | 3 (12.0%) | 2 (18.2%) | 0 (0%) | |
| FK+MMF+PD | 37 (78.7%) | 22 (88.0%) | 9 (81.8%) | 11 (100%) | |
| Sirolimus or everolimus combination | 2 (4.3%) | 0 (0%) | 0 (0%) | 0 (0%) | |
|
|
|||||
| AR | |||||
| Within 1 month post-transplant | 8 (17.0%) | 5 (20.0%) | 2 (18.2%) | 3 (27.3%) | 0.893 |
| Within 3 months post-transplant | 8 (17.0%) | 8 (32.0%) | 3 (27.3%) | 3 (27.3%) | 0.526 |
| Within 6 months post-transplant | 8 (17.0%) | 11 (44.0%) | 4 (36.4%) | 4 (36.4%) | 0.099 |
| Within 12 months post-transplant | 10 (21.3%) | 11 (44.0%) | 4 (36.4%) | 4 (36.4%) | 0.238 |
| During F/U period | 15 (31.9%) | 14 (56.0%) | 7 (63.6%) | 7 (63.6%) | 0.071 |
|
|
|||||
| AR or MVI only | |||||
| Within 1 month post-transplant | 11 (23.4%) | 5 (20.0%) | 3 (27.3%) | 3 (27.3%) | 0.952 |
| Within 3 months post-transplant | 11 (23.4%) | 8 (32.0%) | 6 (54.5%) | 3 (27.3%) | 0.268 |
| Within 6 months post-transplant | 11 (23.4%) | 11 (44.0%) | 7 (63.6%) | 4 (36.4%) | 0.069 |
| Within 12 months post-transplant | 13 (27.7%) | 11 (44.0%) | 7 (63.6%) | 4 (36.4%) | 0.152 |
| During F/U period | 18 (38.3%) | 14 (56.0%) | 10 (90.9%) | 7 (63.6%) | 0.012 |
* Continuous variables are reported as mean±SD or median (interquartile range), and categorical variables are listed as number (%). Kolmogorov-Smirnov test was employed for testing normality assumption.
Abbreviations: Anti-AT1R, anti-angiotensin II type 1 receptor antibodies; AECA, anti-endothelial cell antibodies; ECXM, endothelial cell crossmatch; BMI, body mass index; cPRA, calculated panel reactive antibodies; CsA, cyclosporine A; MMF, mycophenolate mofetil; PD, prednisolone; FK, tacrolimus; AR, acute rejection; MVI, microvascular inflammation.
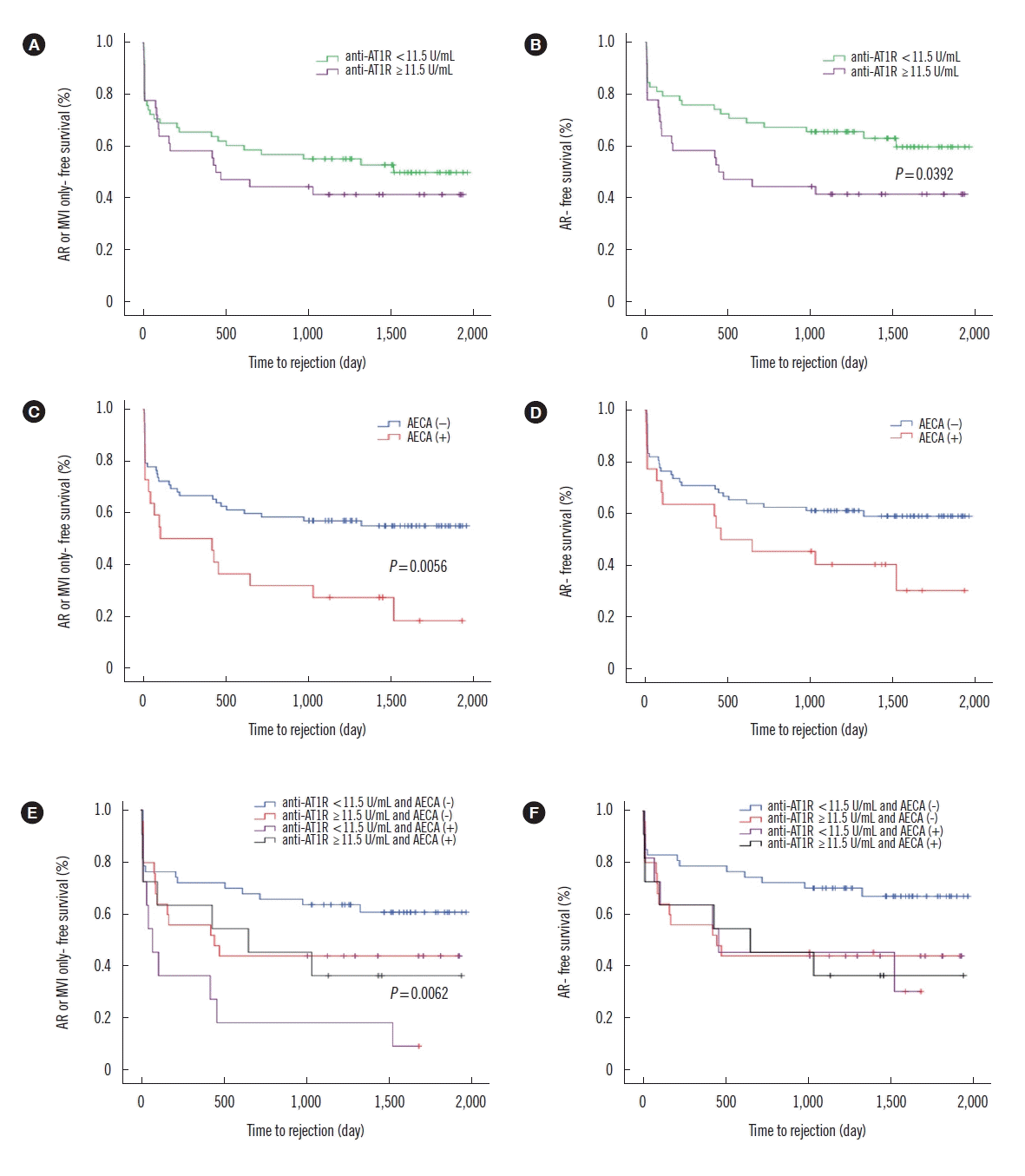 | Fig. 1Clinical outcomes according to the anti-AT1R levels and AECA status using ECXM assay. (A) No significant differences in AR or MVI only free survival rates are seen between recipients with anti-AT1R ≥11.5 U/mL (N=36) and those with anti-AT1R <11.5 U/mL (N=58). (B) Recipients with anti-AT1R ≥11.5 U/mL have a higher risk of AR than those with anti-AT1R <11.5 U/mL (P=0.039). (C) AECA (+) recipients (N=22) have a higher risk of AR or MVI only than AECA (−) recipients (N=72) (P=0.006); (D) There is no significant difference in the AR free survival rates. (E) AECA (+) recipients with anti-AT1R <11.5 U/mL (N=11) have a higher risk of AR or MVI only than other recipients (P=0.006); (F) There are no significant differences in AR free survival rates among the four groups.
Abbreviations: Anti-AT1R, anti-angiotensin II type 1 receptor antibodies; AECA, anti-endothelial cell antibodies; ECXM, endothelial cell crossmatch; AR, acute rejection; MVI, microvascular inflammation.
|
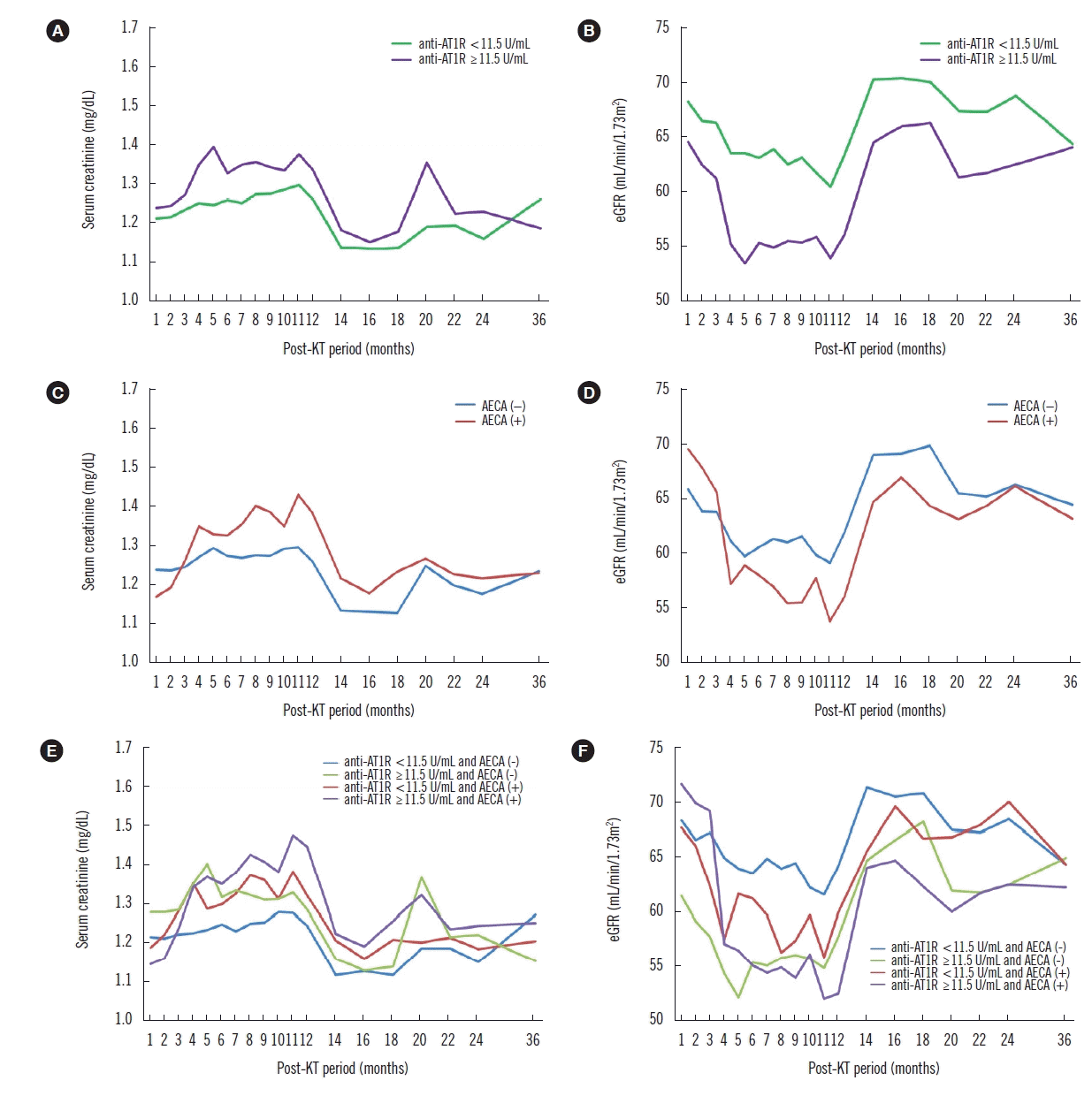 | Fig. 2Effect of anti-AT1R levels and AECA status using ECXM assay on renal function during the post-KT period. Recipients with pre-transplant anti-AT1R ≥11.5 U/mL show significantly lower eGFR (B) but not creatinine levels (A) at 6 and 12 months post KT (P=0.012; P=0.012, respectively), compared with those at one month post-KT. AECA (+) recipients have significantly higher creatinine levels (C) and lower eGFRs (D) at six (P=0.003; P=0.028, respectively) and 12 months (P<0.001; P=0.011, respectively), compared with those at one month post-KT. The change in the pattern of creatinine levels in AECA (+) recipients from one to 12 months post-KT is significantly different compared with that in AECA (−) recipients (P=0.038) (C). AECA (+) recipients with anti-AT1R ≥11.5 U/mL show significantly different changes in the pattern of creatinine levels (E) from one to 12 months post-KT (P=0.045) compared with other recipients, and significantly higher creatinine levels and lower eGFRs (F) at 12 months (P<0.001; P=0.028) compared with those at one month post-KT.
Abbreviations: Anti-AT1R, anti-angiotensin II type 1 receptor antibodies; AECA, anti-endothelial cell antibodies; ECXM, endothelial cell crossmatch; KT, kidney transplantation; eGFR, estimated glomerular filtration rate; MVI, microvascular inflammation.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download