Infection with intestinal parasites occurs worldwide and represents a widespread and serious public health concern in developing countries [
1]. In Korea, intestinal parasitic infections were highly prevalent [
2], but their prevalence has drastically declined [
3]. Currently,
Clonorchis sinensis infection is the most prevalent intestinal parasitic infection in Korea [
3,
4]. With an estimated 932,540 Koreans being infected, the prevalence of clonorchiasis is 1.9% [
4], and it remains highly endemic in riverside areas of Korea [
5]. For the control of clonorchiasis, accurate and rapid diagnosis is essential. Clonorchiasis can be diagnosed on the basis of stool microscopy, imaging, serology, and molecular methods. Serological analysis using enzyme-linked immunosorbent assay (ELISA) is useful for diagnosing clonorchiasis, but it is insensitive for low-burden infection. Although molecular diagnostic techniques are sensitive, they are associated with issues concerning contamination and high cost, which hindered their application in large-scale field surveys [
6-
9]. Stool examination for
C. sinensis eggs is the gold standard for clonorchiasis diagnosis [
10,
11], but it is labor-intensive, requires a high level of technical expertise, and can be insensitive for low-burden infection [
12].
There is an increasing demand for low-complexity, highthroughput, and cost-effective tools to replace labor-intensive microscopic examinations. Accordingly, digital imaging diagnosis based on the morphometric characteristics of parasite eggs has been proposed [
13-
15]. The first study to use an automated stool examination system suggested that an adjusted algorithm should be created for the correct classification of helminth eggs [
13]. Recently, a cuvette-based automated microscopy analyzer, sediMAX 1 (77 Elektronika, Budapest, Hungary), was evaluated for the detection of intestinal parasites, helminths, and protozoa [
14]. The sediMAX 1 analyzer showed perfect performance for the detection of
Dibothriocephalus nihonkaiensis,
Taenia species,
Hymenolepis nana,
Trichuris trichiura,
Ascaris lumbricoides, and
Paragonimus westermani [
14,
15]. However,
C. sinensis and other intestinal parasites prevalent in Korea were not evaluated in these studies. Similarly to sediMAX 1, the newly developed AVE-562 analyzer (AVE Science & Technology Co., Hunan, China) allows vision-based detection of eggs. Here, we evaluated the utility of the AVE-562 analyzer for detecting
C. sinensis eggs in low-burden infections. Additionally, we assessed the performance of this system compared with that of conventional examination for the detection of
C. sinensis eggs in stool samples.
In total, 30 stool samples with a high or low egg count or without eggs (as negative control samples; N= 10 each) were prepared and analyzed. Then, the amount of eggs in high-count formalinized stool samples collected for laboratory quality-control purposes at Chonnam National University Hospital, Gwangju, Korea, from January 2018 to December 2019 was adjusted to 150 eggs/mL. Stool samples were collected with the approval provided by the Institutional Review Board of Chonnam National University Hospital (IRB CNUH-2015-052). All data obtained were anonymized, and no information was used that could lead to patient identification. The need for written consent was waived due to minimal risk to the subjects.
Formalized stool samples within a month from subjects not infected with
C. sinensis served as negative controls. The low-count samples (< 10 eggs/mL) were prepared by mixing 10 µL of high-count samples with 1 mL of negative control samples. Automated vision-based stool examination for egg detection was performed using the AVE-562 analyzer. On adding the formalinized stool samples to the sampling cup, cuvette slides were automatically generated. The internal charge-coupled device camera captured 20 images, which were analyzed using the system software (AVE Science & Technology Co.). The images were reviewed on-screen for the presence of parasites by two experts, each blinded to the other’s findings. After one run of the analyzer, the cuvette contents were re-examined under a Nikon Eclipse Ni-U microscope (Nikon Co., Tokyo, Japan). Conventional stool examination was performed using the formalin-ether concentration (FEC) method [
10]; three slides per sample were observed independently by two experts. The procedure time for conducting one, five, and 10 tests simultaneously was evaluated for both methods. The chi-squared or Fisher’s exact test was used to compare the overall correct identification rates. Student’s
t-test was used to compare the procedure times. The performance (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) of the AVE-562 analyzer was evaluated and compared with that of FEC as a gold standard. All statistical analyses were performed using the GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA), and significance was determined at
P < 0.05.
Of the 10 high-egg-count samples, only four tested positive using the AVE-562 analyzer, whereas all 10 tested positive by the FEC method (
Table 1). Of the 10 low-egg-count samples, none tested positive using the AVE-562 analyzer, and only one tested positive using the FEC method. All negative control samples tested negative using both methods. The overall correct identification rate of the AVE-562 analyzer based on the FEC results was 66.6% (14/21) (
P < 0.001). The sensitivity, specificity PPV, and NPV of the AVE-562 analyzer were 36.4%, 100.0%, 100.0%, and 73.1%, respectively. When we analyzed the seven samples, eggs in which were detected using the FEC method but not using the AVE-562 analyzer, two tested positive on cuvette reexamination, four tested positive by retrospective image observation, and the remaining one was a test failure. Such falsenegatives may be overcome through the acquisition of more images (> 20) or the development of an image-processing software based on neural networks [
16]. The AVE-562 analyzer had a shorter total procedure time than the FEC method (
P = 0.01 for a test,
P = 0.0131 for five tests,
P = 0.0275 for ten tests, respectively) (
Fig. 1). Thus, the AVE-562 analyzer allows rapid testing and is easy to use; however, its diagnostic sensitivity requires improvement.
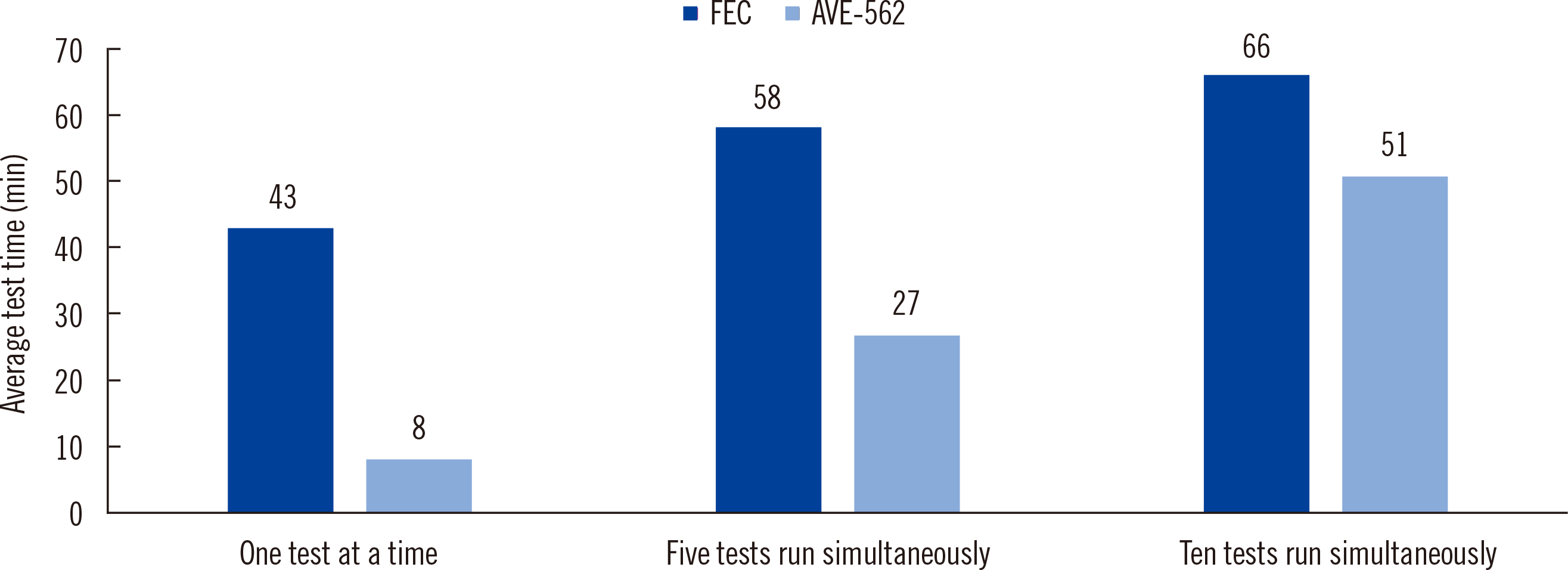 | Fig. 1
Average procedure times for the two methods as a function of the number of samples analyzed simultaneously. The average times required to run one, five, and 10 tests at a time were 8, 27, and 51 minutes, respectively, for the AVE-562 analyzer and 43, 58, and 66 minutes, respectively, for the FEC method. The AVE-562 analyzer showed a significantly shorter total procedure time than the FEC method (P = 0.01 for one test, P = 0.0131 for five tests, P = 0.0275 for ten tests, respectively).
Abbreviation: FEC, formalin-ether concentration.

|
Table 1
Performance of the AVE-562 analyzer for the detection of Clonorchis sinensis eggs
|
N (%) of correct results |
Performance (95% confidence interval)*
|
|
|
|
Positive samples (N=20) |
Negative samples (N=10) |
Subtotal |
Sensitivity |
Specificity |
Positive predictive value |
Negative predictive value |
|
|
High count (N=10) |
Low count (N=10) |
|
AVE-562 |
4 (40%)†
|
0 (0%) |
10 (100%) |
14/21 (66.6%)†
|
36.40% (10.9%-69.2%) |
100% (82.3%-100.0%) |
100% |
73.10% (63.4%-80.9%) |
|
FEC |
10 (100%)†
|
1 (10%) |
10 (100%) |
21/21 (100%)†
|
NA |

Our study had several limitations. First, eggs of intestinal parasites prevalent in Korea other than C. sinensis were not evaluated; this is required to ensure the specificity of the AVE-562 analyzer. Second, we analyzed a relatively small number of samples. However, the AVE-562 analyzer has enough potential to detect C. sinensis eggs in routine screening of stool samples. In addition, images stored by the AVE-562 analyzer can be also useful for educational and quality-control purposes. Overall, our findings indicate that the AVE-562 analyzer enables rapid and convenient stool examination in clinical laboratories, where multiple samples are tested in real-time. However, further improvement is required for the more sensitive and accurate diagnosis of intestinal parasite infections on the basis of vision-based egg detection using the AVE-562 analyzer.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download