INTRODUCTION
Despite improvements in the outcome of multiple myeloma with novel agents, the outcome of advanced Light chain (AL) amyloidosis has not improved significantly. Especially, the outcome of patients with advanced cardiac involvement is dismal. However, the understanding of the pathophysiology of AL amyloidosis has progressed. Moreover, there are active trials investigating new therapeutic options to improve the outcome of AL amyloidosis. Here, I reviewed the advances in both the pathophysiology and treatment of AL amyloidosis.
Go to : 
EPIDEMIOLOGY AND CLINICAL CHARACTERISTICS
Epidemiology of AL amyloidosis varies across reports, but it is within 8–15% annual incidence per million [1, 2]. The prevalence of the disease increases with an increase in age. Mean age at diagnosis is mid 60’s, and there is a predilection for men (55% vs. 45%). In addition, genetic factors contribute to the development of AL amyloidosis and polymorphism in the splice site of CCND1 (rs9344) and SMARCD3 (rs79419269) is strongly related to AL amyloidosis [3]. Malignant B cell clone producfes an immunoglobulin light chain l in 75–80% of cases and k light chains in the remaining cases [4].
Go to : 
PATHOPHYSIOLOGY
Formation of amyloid fibrils
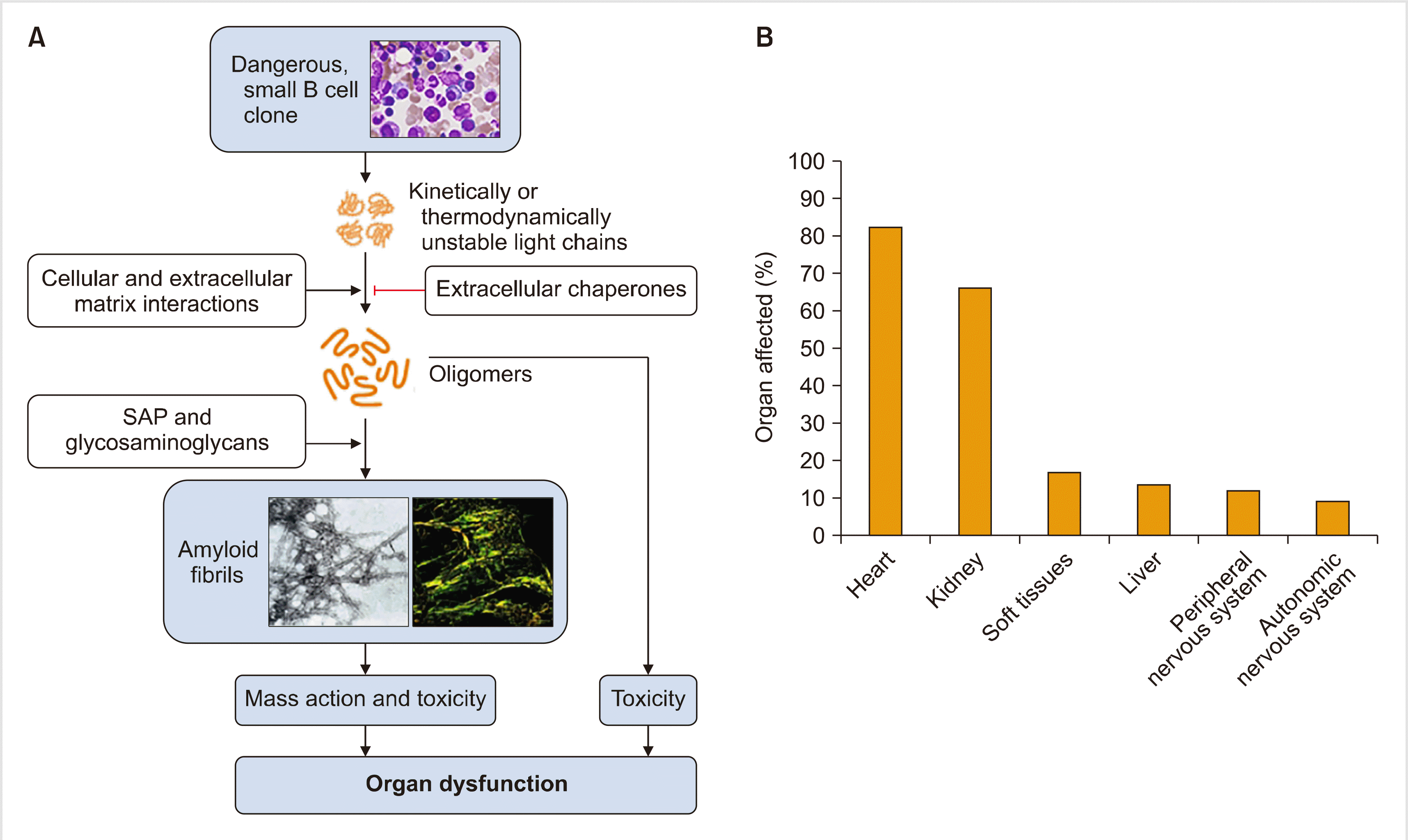
The key process in amyloidosis is the conversion of soluble proteins into insoluble amyloid fibrils that deposit in organs. Protein aggregation is countered by protein homeostasis (proteostasis) that maintains the proteome, and disruption of this balance by any reason contributes to amyloidosis [5]. Overall, -1,600 different proteins play a role in proteostasis, the efficiency of which declines with age. If adequate proteostasis fails, protein aggregation might occur. Somatic mutations in IGLV are frequently found in AL amyloidosis, and they reduce the fold stability of the native protein and moreover, they increase protein dynamics and production of variable light chain domains that favors endoproteolysis and amyloidosis, respectively [6].
Once formed, oligomers of light chains form highly organized amyloid fibrils. The pentraxin serum amyloid P component (SAP) is a circulating plasma protein that is universally present in amyloid deposits owing to its calcium-dependent binding to amyloid fibrils [7]. SAP has been reported to protect amyloid fibrils from degradation. The accumulation of amyloid deposits in parenchymal tissue leads to tissue damage, which causes dysfunction in vital organs. In addition, amyloid fibrils cause cytotoxicity and promote the misfolding of light chains and further oligomer formation (Fig. 1) [8].
Mechanism of cardiac dysfunction
Cardiac dysfunction can result from proteotoxicity of the light chains and amyloid deposits that cause widespread disruption of tissue architecture. Other mechanisms include perturbation of cell membrane by amyloid fibrils, cell toxicity from fibril growth, and the formation of soluble light chain oligomers by amyloid fibrils. Moreover, soluble amyloid light chains, unlike fibrils, induce apoptosis. In addition, it is known that amyloidogenic light chains can induce MAPK signaling, resulting in increased production of reactive oxygen species (ROS), impaired calcium homeostasis, cell dysfunction, and eventually cell death in isolated adult cardiomyocytes [9].
Go to : 
DIAGNOSIS
Diagnostic principles
The diagnostic criteria for AL amyloidosis include the presence of a systemic syndrome, histological documentation of amyloid, evidence of a monoclonal plasma cell disorder, and protein typing that supports the diagnosis. Among systemic syndromes, cardiac involvement is common (60–75%) and of utmost importance in determining the prognosis [10].
Confirming cardiac involvement
Traditionally, a biopsy of bone marrow or abdominal fat is performed to confirm organ involvement. However, it is important to demonstrate clinically meaningful organ involvement such as heart or kidney to decide the treatment. Because the heart is the most commonly affected organ (>60%) and decides the fate of patients, determining heart involvement is the most critical part of the diagnosis. Recent advances in imaging techniques have contributed to the logical diagnostic procedure of determining heart involvement.
The most useful and commonly used method is cardiac magnetic resonance imaging (MRI). Cardiac MRI has high sensitivity (80–100%) and specificity (80–94%) [11, 12]. The finding of late gadolinium enhancement is highly suggestive of the diagnosis of amyloidosis. Along with MRI, scintigraphy with Tc-99m-pyrophosphate (99mTc-PYP) helps to differentiate ATTR (amyloid transthyretin) from AL amyloidosis. PYP scan is positive in almost all cases of ATTR amyloidosis, but it can be positive in some (40%) AL cases. Considering both the presence of monoclonal gammopathy and PYP scan results may help differentially diagnosing amyloidosis involving the heart [13].
However, the gold standard to confirm cardiac involvement is an endomyocardial biopsy, and this should be performed if the results from biochemical and radiological examinations are conflicting. Once a biopsy specimen is obtained, the typing of amyloid is an essential part of the diagnosis. Since MGUS is often present in the aged population, the existence of monoclonal proteins in the serum, urine, or clonal bone marrow plasma cells are not diagnostic for AL type and thus, might be misleading. Hence, the typing of amyloidosis with biopsy specimens is highly recommended. There are three methods of amyloid typing: immunohistochemistry (IHC), immunoelectron microscopy (IEM), and mass spectrometry [14]. IHC is the most commonly used technique in the clinic for determining heart involvement, but IEM examination is highly recommended for an accurate diagnosis. Mass spectrometry is the most sensitive and specific method for diagnosis [15], but there is a gap between the bench and clinic in Korea.
Staging system
The Mayo clinic proposed two prognostic models in 2004 and 2012, respectively. Additionally, there is a European model, which is a modification of the Mayo 2004 model (Table 1) [16-19]. The European model and the Mayo 2012 model showed the best predictive performance in recent validation studies [12].
Table 1
Three prognostic models of AL amyloidosis [12].
| Prognostic model | Criteria | Survival |
|---|---|---|
| Mayo model 2004 [16] | TnT<0.035 μg/L | Stage 1-median 26.4 mo |
| NT-pro-BNP<332 ng/L | Stage 2- median 10.5 mo | |
| BNP<81 ng/L | Stage 3- median 3.5 mo | |
| TnI<0.1 μg/L | ||
| Mayo model 2012 [17] | TnT<0.025 ng/mL | Stage 1- median 94.1 mo |
| NT-pro-BNP<1,800 pg/mL | Stage 2- median 40.3 mo | |
| Serum dFLC<180 mg/dL | Stage 3- median 14 mo | |
| BNP<400 ng/L | Stage 4- median 5.8 mo | |
| TnI- No data | ||
| European model 2015 [18] | TnT<0.035 ng/mL | Stage 1- no death cases |
| NT-pro-BNP<332 pg/mL | Stage 2- 3 yr 52% | |
| BNP<81 ng/L | Stage 3a- 3 yr 55% | |
| TnI<0.1 μg/L | Stage 3b- 3 yr 19% | |
| *Mayo 2004 stage 3 has been subclassified based on NT-pro-BNP<8,500 pg/mL | ||
| Boston university score 2019 [19] | TnT<0.1 ng/mL | Stage 1- median not reached |
| BNP<81 pg/mL | Stage 2- median 9.4 yr | |
| Stage 3a- median 4.3 yr | ||
| Stage 3b- median 1 yr |
Go to : 
TREATMENT
The ultimate treatment goal of AL amyloidosis is the functional recovery of the involved organ. Considering the pathophysiology of AL amyloidosis, eradication of the malignant B cells is a precondition for organ function recovery. However, not all B cell eradicating strategies correlate with organ function recovery. Hence, the treatment strategies are divided into two functional categories as discussed below.
Targeting malignant plasma cells
For patients who are eligible for autologous stem cell transplantation (ASCT), upfront ASCT is deemed to be the standard of care. Eligibility criteria for ASCT include good performance status; preserved cardiac function (cardiac biomarkers below the thresholds), pulmonary function, and hepatic function; and hemodynamic stability (systolic pressure >90 mmHg).
In patients who are not eligible for upfront ASCT, regimens using bortezomib are now considered the standard of care in most cases. Studies have reported that response rates were higher with bortezomib-based regimens than with previous standards of care. The addition of daratumumab to bortezomib-based treatment has been evaluated and but the results have not been published (NCT03201965).
However, high-risk patients represent -20% of AL amyloidosis. Due to advanced cardiac dysfunction or severe heart failure, no treatment regimen can substantially alter the course of the disease in these patients, and they have a very short median survival (around 6 mo). In these patients, removal of the amyloid deposit is necessary to improve the prognosis.
It is noteworthy that a high frequency (40–60%) of chromosome t(11;14) characterizes amyloid B cell clone. A recent trial using venetoclax showed good response in t(11;14) translocated multiple myeloma population [20] suggesting that venetoclax may play a role in the treatment of AL amyloidosis in the future.
Targeting amyloid formation
Monoclonal antibodies that target existing amyloid deposits including SAP have been developed. SAP has been reported to protect amyloid fibrils from degradation. Therefore, SAP is an excellent candidate for amyloid scintigraphy and a target for amyloid-directed immunotherapy.
NEOD001 targets amyloid fibrils. In the early phase trial, NEOD001 showed a favorable safety profile and a fairly good response rate. However, the phase 3 trial failed to confirm the clinical benefit of NEOD001 in cases with cardiac involvement [4, 21].
Similarly, murine monoclonal antibody 11-1F4 targets the human light chain-related fibrils. It showed good efficacy with minimal toxicity in the phase 1 trial [22]. Further clinical trials on11-1F4 are currently being planned.
Theoretically, the anti-SAP antibody is applicable to all types of amyloidosis. Dezamizumab binds to SAP and induces a macrophage response that triggers the rapid removal of SAP. The first phase 1 trial showed amyloid removal from the liver, spleen, and kidneys when SAP scintigraphy was performed [23]. Although its trial has been halted in ATTR amyloidosis with cardiac involvement due to risk-benefit calculation [24], evaluation of this antibody in AL cardiac amyloidosis is pending and warranted.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download