This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The aim of this study was to estimate the prevalence of the beta thalassemia trait (BTT) and differentiate it from iron-deficiency anemia (IDA) among blood donors.
Methods
A total of 1,000 samples from blood donors were subjected to complete hemogram with red blood cell indices. Further, Mentzer index (MI) was calculated for samples with mean corpuscular volume (MCV) below 80 fL and mean corpuscular hemoglobin (MCH) below 27 pg. Samples with Mentzer index <12 were subjected to naked-eye single-tube red cell osmotic fragility test (NESTROFT) followed by hemoglobin electrophoresis in positive cases. Serum ferritin was assessed in NESTROFT-negative cases.
Results
The prevalence of BTT among blood donors was 3.7% and that of microcytosis among donors was 8.6%. The prevalence of BTT among microcytic donors was 41.8% while that among those with IDA was 11.6%. A value of MI <13 was highly sensitive in the diagnosis of BTT. MI >13 was found to have both high specificity and high sensitivity for diagnosing IDA.
Conclusion
A moderately high prevalence of BTT was observed among blood donors. Presently, no screening program is mandatory for screening of BTT among blood donors. Indices like MCV, MCH, and Mentzer Index were thus found to be effective to screen for BTT and IDA among blood donors, and NESTROFT was a cost-effective mass screening method to differentiate BTT and IDA.
Go to :

Keywords: Beta thalassemia trait, Blood donors, Mentzer index, NESTROFT, Iron deficiency anemia
INTRODUCTION
Thalassemias are a group of anemias resulting from genetic defects in the production of one or more of globin polypeptide chains of hemoglobin. These disorders lead to disruption in the production of alpha- or beta-globin polypeptide chains. Imbalance in the globin chain ratio and precipitation of unpaired chains lead to ineffective erythropoiesis and hemolytic anemia [
1].
Thalassemias are inherited in an autosomal recessive pattern. The prevalence of thalassemias is particularly high in the Mediterranean region, the Indian subcontinent, Southeast Asia, and West Africa [
2]. Clinically, alpha and beta thalassemia may occur in varied genetic forms. Beta thalassemia trait (BTT) is the carrier state of beta thalassemia major, a disease that has grave economic implications for a developing country like India.
Worldwide, about 56,000 live births are diagnosed with a major thalassemia disorder each year; of these, approximately 30,000 are affected with beta thalassemia major [
3]. The global prevalence of beta thalassemia carriers has been reported to be 1.5% [
4]. The highest frequency is reported in Cyprus (14%) followed by Sardinia (10.3%) [
5]. In India, the overall frequency is 4% [
6]. Certain communities like Sindhis, Muslims, Kutchi Bhanushali, and some tribal groups have higher prevalence rates ranging from 8% to 10% [
7]. Early diagnosis of BTT is vital to control the rising incidence of beta thalassemia major, a transfusion-dependent anemia associated with a wide gamut of complications. Screening for carriers of beta thalassemia at an appropriate age is an important tool to prevent thalassemia from occurring in children [
8].
The present study aimed to determine the prevalence of BTT among blood donors and also to differentiate it from iron-deficiency anemia (IDA) in the healthy blood donor population using simple screening tools such as the Mentzer index (MI) and naked-eye single-tube red cell osmotic fragility test (NESTROFT).
Go to :

MATERIALS AND METHODS
This was a prospective observational study conducted at the Department of Transfusion Medicine, in collaboration with the Department of Pathology, over a period of one-and- half years. The study was approved by the Institutional Ethics Committee of the institution, and written informed consent was obtained from the donors. A total of 1,000 samples of both voluntary and replacement blood donors was studied to determine the prevalence of BTT. Donors deferred due to low hemoglobin level (Hb<12.5 g/dL) were also included in the study.
The inclusion criteria were voluntary and replacement blood donors aged 18–40 years weighing more than 45 kg and willing to participate in the study. Only first-time donors and those with a last donation more than two years prior to the study, but with a hemoglobin level above 12.5 gm/dL, were enrolled. First-time donors deferred due to low hemoglobin levels (below 12.5 gm/dL) were also included. Regular blood donors and donors deferred due to other reasons except low hemoglobin were excluded from the study. Blood samples of all donors eligible for the study were taken after donation of blood; blood samples of deferred donors were also taken. EDTA was used for obtaining a complete hemogram. Samples were analyzed using an automated cell counter within 24 hours of donation or sample collection.
Mean corpuscular volume (MCV) less than 80 fL was considered as microcytosis. MI was calculated by dividing the MCV by the red blood cell count for all the samples having MCV <80 fL and MCH <27 pg. The MI of each such sample was recorded, analyzed, and grouped under the following categories: MI >14, suggestive of IDA; MI 12–14, intermediate; MI <12, suggestive of BTT.
Samples having MI <12 and those with intermediate MI (range, 12–14) were subjected to NESTROFT [
9]. Briefly, 2 mL of 0.36% buffered saline was taken in a test tube, and 2 mL of distilled water was taken in another test tube. After addition of 20 mL of anticoagulated whole blood in each tube, both tubes were mixed well and left undisturbed for half an hour at room temperature. Subsequently, the contents were shaken and the tubes were held against a white paper on which a thin black line was drawn and observations were made regarding the visibility of the black line (yes or no). The tubes were then left undisturbed a further few hours and the contents again checked for hemolysis. The results were interpreted as NESTROFT positive or NESTROFT negative.
NESTROFT positive: Cases in which the black line was not visible or was hazy after 30 minutes of incubation at room temperature were considered positive. After prolonged incubation at room temperature, the presence of red cell sediments at the bottom of the tube and colorless saline in the remaining part of tube was observed.
NESTROFT negative: Cases in which the black line was clearly visible through the contents of the tube after 30 minutes of incubation at room temperature were considered negative. After prolonged incubation at room temperature, the presence of cell lysis in the form of uniformly pink distilled water, with no sedimentation of red cells at the bottom of the tube, was observed.
All the NESTROFT-positive cases were subjected to hemoglobin electrophoresis at alkaline pH 8.6 and assessed for the presence of a band in the A2 region. All samples showing band in the A2 region on hemoglobin electrophoresis were considered as having a BTT.
Serum ferritin was assessed using enzyme-linked immunosorbent assay (ELISA; Orgentec Diagnostica GmbH) in cases where MI >14 and where there was indeterminate MI (range, 12–14) as well as in all NESTROFT-negative cases. This was also assessed in those with RBC indices highly suggestive of BTT and were NESTROFT positive, but had no band in the A2 region on hemoglobin electrophoresis. This was done to rule out the concomitant presence of IDA.
Levels of serum ferritin below 15 ng/mL were considered as IDA (
Fig. 1) [
10].
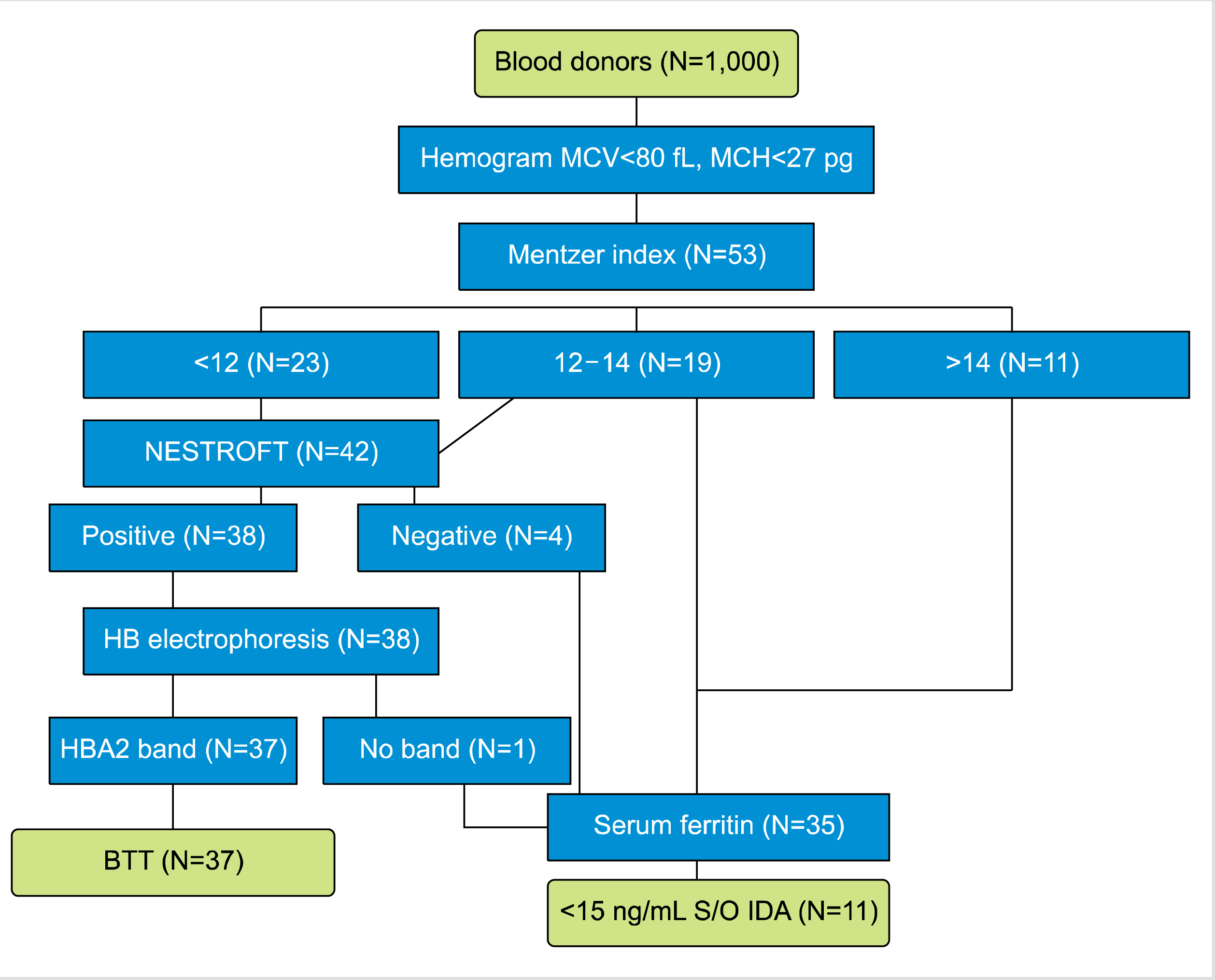 | Fig. 1
Flow chart for defining of BTT and IDA and the number of cases in the study. Samples with normal serum ferritin and distinct HbA2 band on electrophoresis were defined as having BTT. Samples with serum ferritin below 15 ng/mL were defined as having IDA [ 10]. Abbreviations: BTT, beta thalassemia trait; IDA, iron-deficiency anemia; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume.

|
Statistical analysis
SPSS (SPSS Inc., Chicago, IL, USA) for Windows version 17.0 was used for data analysis. Prevalence rates were calculated and compared according to the type of donor, age group, gender, place of residence, religion, and presence or absence of family history of consanguinity and family history of thalassemia. Continuous data were analyzed in the form of mean and standard deviation. Differences were evaluated using the Chi-square test and Student’s t-test. A P-value of <0.05 was considered statistically significant.
Go to :

RESULTS
Out of 1,000 blood donors, 885 (88.5%) volunteered while 115 (11.5%) were replacement donors. Among voluntary donors, 815 (92.1%) were males and 70 (7.9%) were females. There were 102 (88.69%) males and 13 (11.31%) females among replacement donors. Majority of donors (941, 94.1%) were accepted donors while 59 (5.9%) were deferred donors. Of the 59 deferred donors, 42 (71.2%) were males and 17 (28.8%) were females. The mean age of donors was 25.3 years, and median age was 24 years. The maximum number of donors (636, 63.6%) were in the age group of 18–25 years and least (2.9%) were in the age group of 36–40 years. The prevalence of BTT among blood donors is shown in
Table 1.
Table 1
Prevalence of BTT among blood donors.
|
Prevalence of BTT (%) |
P
|
|
Overall |
3.7 |
|
|
Voluntary donors |
2.48 |
˂0.01 |
|
Replacement donors |
13.04 |
|
|
Accepted donors |
1.6 |
˂0.01 |
|
Deferred donors |
37.2 |
|
|
Males |
3.38 |
˃0.05 |
|
Females |
7.23 |
|
|
18–30 yr |
4.3 |
˃0.01 |
|
30–40 yr |
0.6 |
|
|
Urban background |
2.8 |
˃0.05 |
|
Rural background |
4.8 |
|
|
Consanguinity |
64.2 |
˂0.01 |
|
No consanguinity |
2.83 |
|

The overall prevalence of BTT among blood donors was 3.7%. The prevalence of BTT varied from 1.53% to 4.25% across various religious groups. The majority of the donors were from Punjab, Chandigarh, and Haryana, in descending order. The highest prevalence of BTT was found in Delhi (8.6%) followed by Punjab (5.2%). Chandigarh had a prevalence of 2.2%, while that in Haryana was 2.7%.
Overall prevalence of microcytosis in blood donors was found to be 8.6%. Prevalence of microcytosis in voluntary donors was 7.2%, while it was 19.1% in replacement donors (P<0.01). Of the 941 accepted donors, 45 (4.8%) were found to be microcytic (MCV<80 fL). Forty-one (69.4%) out of 59 deferred donors were found to be microcytic. The difference was highly statistically significant (P<0.01).
Of the 86 donors with microcytosis, 41 (47.6%) were found to be anemic (Hb<12.5 g/dL) while 45 (52.4%) microcytic donors had hemoglobin levels above 12.5 g/dL. The prevalence of BTT among microcytic donors was 41.8% and that of IDA among microcytic donors was 11.6% (
Table 2). Out of the 86 microcytic donors, MI was found to be applicable (MCV<80 fL and MCH<27 pg) in 53 donors. Out of these 53 donors, 23 donors had MI <12, 19 donors had MI=12–14, and 11 donors had MI >14 (
Table 3).
Table 2
Prevalence and type of anemia among microcytic blood donors.
|
Microcytic donors |
86 |
Hb>12.5 g/dL |
45 (52.4%) |
Normal |
30 |
|
BTT |
15 |
|
Hb<12.5 g/dL |
41 (47.6%) |
BTT |
21 |
|
IDA |
10 |
|
Both |
1 |
|
None |
9 |

Table 3
Association of different MI values with type of anemia.
|
BTT |
IDA |
Others |
Total |
|
MI<12 |
23 |
0 |
0 |
23 |
|
MI 12–14 |
14 |
1 |
4 |
19 |
|
MI>14 |
0 |
10 |
1 |
11 |
|
Total |
37 |
11 |
5 |
53 |

On further analysis of MI in the indeterminate range of 12–14, it was observed that MI <13 was highly sensitive for the diagnosis of BTT. While specificity of MI <13 was 68.7%, its sensitivity was 100%. Its positive predictive value was 88% and negative predictive value was 100%. MI >13 was found to have both high specificity and sensitivity for diagnosing IDA. It showed a sensitivity of 90.9% and a specificity of 97.6%. The positive predictive value for MI >13 was 90.9% and negative predictive value was 97.6%.
Analysis and comparison of the mean values of various hematological parameters in BTT and non-BTT donors were carried out. Mean RBC count was significantly higher in the BTT group (5.89×10
6/mL) compared to the non-BTT group (4.68×10
6/mL;
P<0.0001), while mean hemoglobin (12.3 g/dL) was slightly less in BTT cases when compared to non-BTT cases (13.9 g/dL;
P<0.001). Similarly MCV, MCH, and MCHC were significantly reduced in BTT cases when compared to non-BTT cases (
P<0.0001), as shown in
Table 4.
Table 4
Comparison of hematological parameters in donors with BTT and non-BTT.
|
Parameter |
BTT (N=37) |
Non-BTT (N=963) |
P
|
|
Hb (g/dL) |
12.3±0.57 |
13.9±1.07 |
<0.0001 |
|
MCV (fL) |
70.4±2.84 |
87.4±6.13 |
<0.0001 |
|
MCH (pg) |
23.7±2.43 |
29.7±1.65 |
<0.0001 |
|
MCHC (%) |
30.2±0.81 |
32.1±1.48 |
<0.0001 |
|
TLC (×103/mL) |
6.26±1.86 |
6.12±1.77 |
0.6376 |
|
RBC (×106/mL) |
5.89±0.34 |
4.68±0.47 |
<0.0001 |
|
PCV (%) |
38.9±2.02 |
42.1±3.28 |
<0.0001 |
|
RDWc (%) |
14.6±0.66 |
14.5±0.43 |
0.1756 |
|
Platelets (×103/mL) |
223±54.2 |
250±67.4 |
0.163 |

Mean hemoglobin was significantly lowered in IDA (11.5 g/dL) with respect to that in BTT (12.3 g/dL;
P<0.001), as shown in
Table 5. MCV and MCH means were significantly reduced in BTT as compared to IDA (
P<0.0001). RBC count was significantly higher in BTT (5.89×10
6/mL) as compared to IDA (4.60×10
6/mL;
P<0.0001). Another significant observation was made in the difference of means of RDWc between BTT and IDA. RDWc mean was significantly higher in IDA (16.1%) as compared to RDWc mean in BTT (14.6%;
P<0.0001). High RBC count and low hemoglobin values were significantly and positively associated with the incidence of BTT.
Table 5
Comparison of hematological parameters in donors with BTT and IDA.
|
Parameter |
BTT (N=37) |
IDA (N=11) |
P
|
|
Hb (g/dL) |
12.3±0.57 |
11.5±0.56 |
<0.001 |
|
MCV (fL) |
70.4±2.84 |
76.4±1.44 |
<0.0001 |
|
MCH (pg) |
23.7±2.43 |
27.4±1.12 |
<0.0001 |
|
MCHC (%) |
30.2±0.81 |
31.2±1.67 |
0.0085 |
|
TLC (×103/mL) |
6.26±1.86 |
5.90±1.94 |
0.5794 |
|
RBC (106/mL) |
5.89±0.34 |
4.60±0.53 |
<0.0001 |
|
PCV (%) |
38.9±2.02 |
35.1±1.72 |
<0.0001 |
|
RDWc (%) |
14.6±0.66 |
16.1±1.36 |
<0.0001 |
|
Platelets (×103/mL) |
223±54.2 |
192±19.1 |
0.07 |
|
MI |
11.4±0.75 |
17.0±2.05 |
<0.0001 |
|
S. ferritin (ng/mL) |
150±73.8 (N=14) |
12.9±1.13 |
<0.0001 |

NESTROFT was found to be applicable in 42 cases, out of which 38 were positive and 4 were negative. One case, out of 38 NESTROFT-positive cases, was found to be negative on Hb electrophoresis. Only positive predictive value for NESTROFT could be derived which was 97.3%.
Go to :

DISCUSSION
The overall prevalence of BTT in our donor population was 3.7%. Other studies from India have shown varied prevalence of BTT ranging from 1.53% to 9.59% [
6,
11-
16]. A study conducted in school children found the prevalence of BTT to be 4.05% [
6]. Another study from the southern part of India conducted in pregnant females reported a prevalence of 8.5% [
13]. India being a country with vast ethnic diversity could be the reason for the wide variation in prevalence. Moreover, target groups and methods employed for estimation of prevalence were also different in these studies.
In a study conducted on blood donors in Malaysia, the prevalence of BTT was reported to be 5% [
17]. In another study conducted in Egyptian blood donors to differentiate between BTT and IDA, the prevalence of BTT was found to be 6% [
18]. A literature report from Pakistan found 73 (14.5%) out of 503 subjects to be beta thalassemia carriers, using NESTROFT as differentiating index between BTT and IDA. This discrepancy in results could be due to the different study populations as the authors included only subjects with microcytosis [
19].
We found a high prevalence of BTT in the age group of 25–30 years (5.4%) followed by 18–25 years (3.9%). Some studies have reported comparable prevalence across all age groups [
12]. Our study population comprised 83.7% donors younger than 30 years of age; hence, prevalence in other age groups could not be ascertained. Although 7.23% of females were carriers of BTT compared to 3.38% males, we do not generalize these figures to the whole population due to small number of female donors in our study.
A highly significant difference was found in prevalence among deferred donors (37.2%) in comparison to accepted donors (1.6%). The mean hemoglobin level of donors with BTT was 12.3%, which justifies the observation of using the cutoff for accepting donation as 12.5 g/dL. Wide variation was present between frequencies of BTT according to the states of residence of the donor, but these were not found to be statistically significant (
P>0.05). State-wise prevalence for the state of Punjab (5.2%) which represented 36.5% of our study population was comparable with the study conducted by Kumar
et al. (4.4%) and Mohanty
et al. (3.96%) [
16,
11]. A higher prevalence in Punjab has been reported by Chatterjee
et al. (11.79%) [
12]. They used Bayesian estimates in assessing the prevalence of BTT. Our results were similar to those of Kumar
et al. [
16] in terms of BTT prevalence in Haryana and Himachal Pradesh.
We found a significant association between a history of consanguinity and BTT, but other authors have reported different results. Our finding of a significant association between the two is likely to be a result of the concomitant presence of associated family history of thalassemia in one-third of the donors, owing to the history of consanguineous marriage in the family. Religion-wise prevalence did not show any significant difference, as reported in other studies [
11,
12,
16].
In our study population, 8.6% of the blood donors were found to be microcytic. Significant association was found between replacement donors and prevalence of microcytosis and also between deferred donors and microcytosis (
P< 0.01). Another significant observation was that 47.6% of the 86 donors with microcytosis were found to be anemic, with 24.4% having IDA, 51.2% having BTT, and 2.4% having concomitant BTT and IDA; in 21.9% donors, neither BTT nor IDA could be confirmed. In a study from the northern part of India, the authors found prevalence of microcytosis in blood donors to be 5.4%, and out of these microcytic donors they reported 52% as having IDA, 36% as having BTT, 8% as having both the conditions and, 4% as having neither condition [
14]. The majority of our donors belonged to plains of Punjab and Haryana, where IDA is less prevalent as compared to the donors from hilly regions of India, like Uttarakhand, where the prevalence of IDA is more. Another recent study from Gujarat reported 14.75% prevalence of microcytosis in blood donors [
15]. Soliman
et al. [
18] reported prevalence of microcytosis to be 10.5% in healthy Egyptian adult blood donors.
The hematological parameters of BTT donors from our study were comparable with those found in other studies, although variations existed in hematological parameters of donors with IDA in our study and the other three studies, which could be due to the small sample size of donors with IDA in our study [
14,
18,
19].
We evaluated MI cut-off value of 13 as a discriminating factor to differentiate between cases of BTT and IDA. MI <13 was found to be significantly associated with BTT, with 100% sensitivity and 68.7% specificity. MI >13 was found to be significantly associated with IDA, with a specificity of 97.6% and sensitivity of 90.9%. Tiwari
et al. [
14] found sensitivity and specificity of 100% and 72%, respectively, in case of IDA and 72% and 100%, respectively, in case of BTT. In case of NESTROFT, we could only derive the positive predictive value, due to our study limitation, and found it to be 97.3%.
High-performance liquid chromatography (HPLC) is used to screen patients for hemoglobin disorders. In a large prospective study from eastern part of India conducted over a period of 10 years, BTT was the most common hemoglobin abnormality (4.6%) [
20]. Some authors have recommended a screening algorithm for blood donors that includes HPLC analysis of microcytic donors with plasma ferritin levels above 15 ng/mL [
21]. However, we could not perform HPLC in our donor population.
Our study has a few limitations. According to our study methodology, we could not perform Hb electrophoresis in all the cases in which NESTROFT was done due to which performance characteristics of NESTROFT could not be evaluated and compared. Moreover, we could not estimate HbA2 levels by HPLC, and DNA profiling could not be done due to financial constraints and lack of infrastructure.
In conclusion, we found moderately high prevalence of BTT among the blood donor population, suggestive of an increased risk of beta thalassemia major incidence in the near future. Currently, no screening program is mandatory for screening of BTT among blood donors, and donor screening is only done for anemia using hemoglobin estimation in blood banks. Based on the findings of this study, screening of blood donors for BTT and differentiating it from IDA is proposed using indices like MCV, MCH, MI, and NESTROFT, which have the advantage of being cost-effective and, thus, an advantage for developing countries like India where BTT and IDA are prevalent. An algorithmic approach can be devised to identify blood donors with BTT and differentiate it from IDA so that the blood donor is appropriately guided for counseling or iron therapy, respectively.
Go to :

ACKNOWLEDGMENTS
The study was approved by the institutional research and ethics committee, and informed written consent was taken from all blood donors who participated in the study.
Go to :






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download