INTRODUCTION
Chronic myeloid leukemia (CML) is a rare disease characterized by the presence of the Philadelphia chromosome (Ph+) [
1]. In 2001, the Food and Drug Administration (FDA) approved imatinib mesylate (Glivec, Novartis, Klybeckstrasse, Basel, Switzerland), a first-generation
BCR-ABL tyrosine- kinase inhibitor (TKI), for the treatment of CML on the basis of the results of two phase 2 studies showing better hematologic and cytogenetic responses in both chronic (CP) and accelerated (AP) phases. Based on the result of the phase 3 IRIS trial, imatinib became the new standard of care for first-line therapy [
2,
3]. Imatinib dramatically changed the prognosis of CML patients, ensuring a life expectancy comparable to that of the general population, with deaths more likely due to comorbidities than due to CML [
4]. Despite this, most patients require continuing imatinib treatment lifelong because of the persistence of quiescent leukemic clones [
5]. The expiration date of market patents vary among countries, and bioequivalence data are sufficient to obtain approval for generic drugs from regulatory entities. In particular, a difference in bioavailability in the range of 80–125% for branded imatinib (Glivec) is considered acceptable [
6]. Since 2012, generic formulations of imatinib were approved in Europe, Canada, and United States on the basis of data showing bioequivalence between generic and branded imatinib [
7-
9]. In Italy, the first generic imatinib was approved by Agenzia Italiana del Farmaco (AIFA) in January 2017. However, some formulations were available in other countries before official patent expiration. Despite this, there is a lack of solid data on the efficacy of generic imatinib. In the literature, only small, retrospective studies, conducted in countries with easier approval rules, are available. Some case reports documented loss of molecular and cytogenetic responses with risk of progression from CP to AP after the switch to generic imatinib, which was reverted with the reintroduction of the branded formulation in most cases [
10-
12]. Saavedra
et al. [
13] reported four out of eight patients who switched to generic imatinib and lost cytogenetic response within three to four months since the switch, and all the eight patients had to switch to second-generation TKI due to disease progression or AEs. In another prospective single-center Iraqi study among 126 CML patients in the CP who were first treated with branded imatinib and then switched to generic imatinib, many patients lost their hematologic response and had problems with tolerability; all patients were switched back to the branded imatinib [
14]. Conversely, in a retrospective analysis from Latvia, second-generation TKIs were not necessary because no disease progression was observed after switch to generic imatinib in 24 months [
15]. Moreover, in a cohort of 58 patients who switched from patented to generic imatinib, the progression-free survival rate at 12 months was 92%, with a favorable safety profile [
16]. Many of these studies were sponsored by pharmaceutical companies and were performed in countries with less stringent quality controls than in Western Europe.
However, owing to these contrasting results, assessing the safety and efficacy of available generic formulations is of paramount importance. Furthermore, some patient associations and clinicians are doubtful about a switch to the generic one for fear of disease worsening and higher side effects [
17-
19]. For these reasons, it is necessary to further assess the efficacy and safety of new generic formulations in larger and prospective trials.
Go to :

MATERIALS AND METHODS
This is an observational, multicenter, retro-prospective analysis of patients with CML in the CP with stable disease [defined as at least 18 months of complete cytogenetic response (CCyR) and 36 months of treatment with branded imatinib] who had their treatment switched from branded to generic imatinib since January 2017 in 12 Italian Institutes belonging to the REL (Lombardy Hematological Network). Previous treatments with interferon or oncocarbide were allowed if administered for a maximum of one month for cytoreductive purpose. Patients in the AP or BP at the time of enrollment were excluded, as well as patients the in second or subsequent lines of treatment. Five manufacturers of generic imatinib were used during this period: Accord, Sandoz, Teva, Dr. Reddy, Mylan; accord accounted for more than 50% of total usage. We analyzed the variation in quantitative PCR values, considering BCR/ABL cDNA copies/≥10,000 ABL copies corrected by the International Standard (IS). Three PCR values were considered in a period of 12 months before the switch and 3 values in the 12 months after the switch (this period was then modified in 20 months considering that, in clinical practice, patients with stable disease were often evaluated for PCR with a six-month interval). PCR tests were performed at each center. Molecular responses were assessed for each PCR value and defined as follows: molecular response (MR) 3 if transcript levels were ≤0.1% BCR-ABL/ABL, MR4 if ≤0.01% BCR-ABL/ABL, MR4.5 if ≤0.0032%, and MR5 if ≤0.001%. We evaluated and compared the median PCR values before and after the switch as well as the proportion of patients who maintained, improved, or worsened their molecular response, and the AEs encountered by the patients with branded and generic formulations; AEs were recorded according to the 3 main AEs declared by the patient and registered by the treating physician. AEs were graded according to the Common Terminology Criteria for AEs (CTCAE) 4.0. Wilcoxon non-parametric test for individual paired data was used to compare the median number of pre-switch copies with the median number of post-switch copies. McNemar non-parametric test for paired proportions was used to compare the proportions of patients with AEs. A 5% significance level was considered for two-sided tests.
The study was conducted in accordance with the Declaration of Helsinki and the rules of the Good Clinical Practice. The study protocol was approved by the Ethics Committee of the Coordinating center and the respective Ethics Committee for each center; patients’ data were anonymized and replaced by a unique identification code.
Go to :

DISCUSSION
Our study involved 200 patients from 12 centers in Lombardia, Italy, affected by CP-CML on first-line treatment with branded imatinib, which was switched to generic imatinib. Patients should have a stable disease, defined as a CCyR for at least 18 months and treatment with imatinib for at least 36 months, to avoid possible confounding factors in assessing the response to treatment after switching. Moreover, after 36 months of treatment, most side effects maybe stable, allowing a more precise report of relevant side effects.
In our study, we observed a significant improvement in PCR transcript values after the switch to generic imatinib. We hypothesized that this may be related to a longer period of treatment (one year after the switch to generic) rather than to a greater efficacy of generic imatinib. This is in contrast to the analysis presented at the ASH 2015 congress by Klil-Drori
et al., [
20] which showed a non-significant worsening (a 1-log increase) of the transcript levels measured by quantitative PCR in a prospective series of 38 patients. In our series, we observed that 23% of the patients (N=46) had an increase in PCR transcript levels, although not substantial in most cases (<0.5 log), which did not affect the clinical decisions. In fact, most patients maintained or even improved their MR (94.5%). Only 11 patients (5.5%) experienced a worsening in their PCR transcript values, although in the range of a stable disease. These findings are consistent with those already reported in a large Polish database showing 89% of stability or further improvement of molecular responses and 11% of patients with worsening response after 12 months of treatment with the generic form [
21]. In a retrospective cohort of 40 patients, Ćojbašić
et al. [
22] reported sustained MR in 72.5% and 12.5% of molecular improvement. This is the study with the longest follow-up, reporting 93.8% of patients alive at 10 years. In contrast, a Canadian prospective matched cohort study analyzed the rate of persistence with generic and branded imatinib: a 3-year rate of persistence with the generic was significantly inferior to that of the branded imatinib (72.8% vs. 88.2%;
P=0.03), with a probability of switching, which was two-fold higher for generic than for branded imatinib. Among patients who opted for the generic drug, 25 (69.5%) had to switch for intolerance and 12 (33.3%) for resistance [
23]. With increasing data demonstrating comparable efficacy of generic imatinib, it is reasonable to expect that similar data may be obtained in case of discontinuation of the generic treatment.
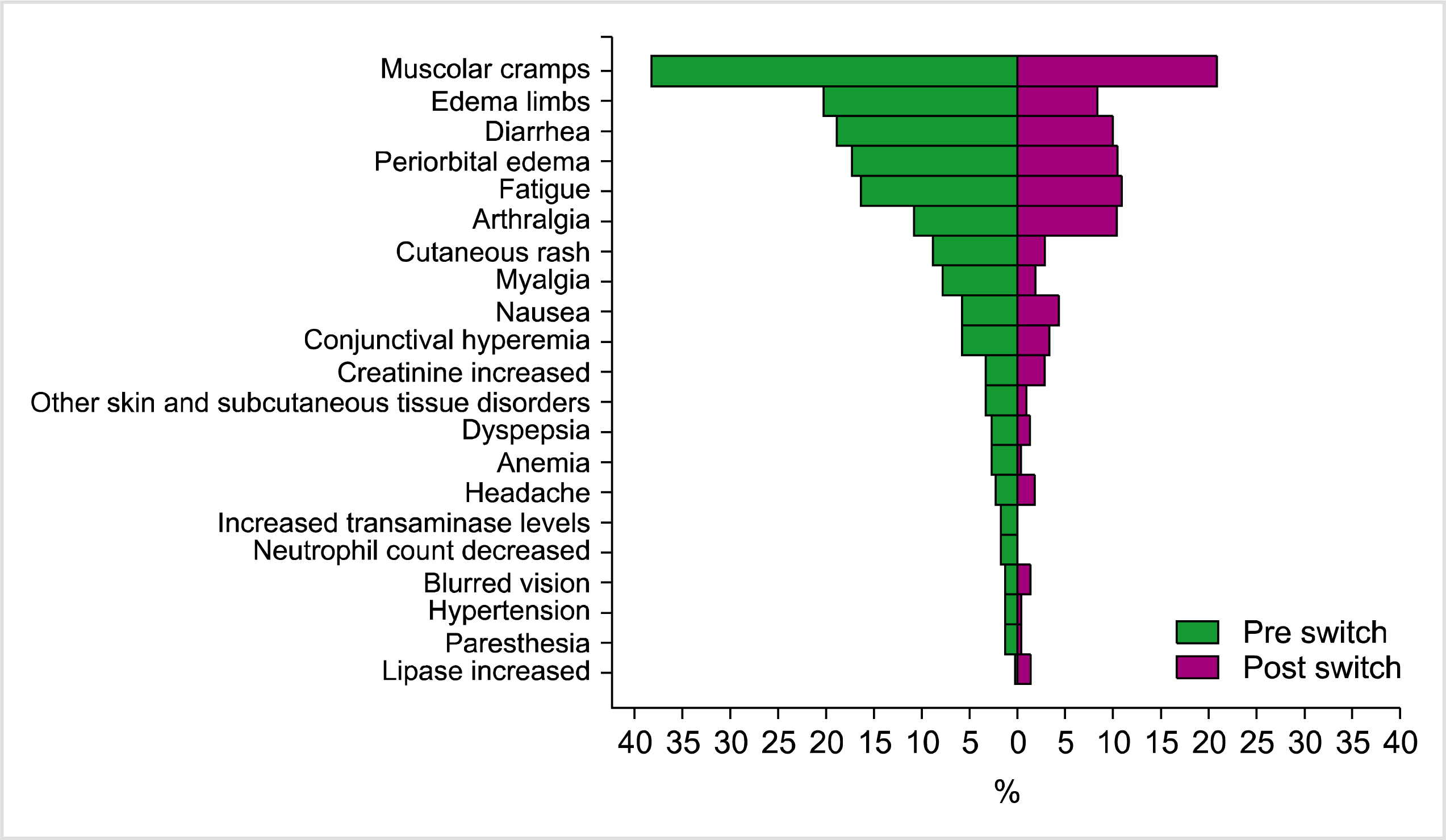
The AEs reported in our study are comparable to those for branded imatinib pertaining to the type and intensity [
3]. Rather, the percentage of reported AEs was lower for generic imatinib, including osteoarticular pain, muscle cramps, and fatigue, with lower rates of G3-G4 events reported after the switch to the generic one (
Table 2 and
Fig. 2). However, some bias may have influenced the safety analysis: first, safety data were collected directly from clinicians and no ad-hoc questionnaire on AEs or quality of life was planned, possibly causing an underestimation of the AEs. Moreover, the fact that patients who switched to generic imatinib were treated for 1 more year could have contributed to a better tolerability of generic imatinib. Despite a general reduction in AEs, some patients reported concerns and fears about the introduction of the generic form of imatinib and thus could be particularly sensitive to any AE associated with generic imatinib. Another explanation of this could be an increased bioavailability of imatinib. A small proportion of patients reported the onset of new AEs after the switch, as reported in
Table 2 and
Fig. 2. However, this analysis is limited by the short observation period before the switch. Owing to intolerance, three patients had to switch back to branded imatinib in our study. Another Italian observational study on 294 patients treated for at least 6 months with branded imatinib and then switched to the generic, addressed the question on generic imatinib safety and showed that 17% of the patients reported worsening or new-onset AEs, while 6 (17.7%) patients had to revert to the branded drug [
24].
We used five types of generic drugs according to individual hospital licenses at the time of patient enrollment. Direct comparison among the different generics was not performed because 22% of the patients (N=44) received 2 to 3 types of different generics, which makes this analysis unreliable. Moreover, our study was aimed to address this question. Small differences in bioequivalence may exist, but they seem not to affect the clinical outcomes. In fact, the FDA, EMA, and AIFA requested that kinetic profiles of generic drugs fall within a range of 80-125% of absorption rate with regard to that of the reference drug, assuming that these small differences in kinetic profile should not affect outcome [
6]. Generic drugs available in Italy are approved on this basis.
In our study, we did not analyze the efficacy and safety of upfront generic imatinib on newly diagnosed CML. Eskazan
et al. [
25] reported a similar percentage of CCyR and major MR after six months in 2 cohorts of patients treated with brand and generic imatinib, respectively. Moreover, achieving major MR at 6 months had a prognostic relevance for branded imatinib [
26]. In another Algerian retrospective study on 355 patients treated with CIPLA imatinib, 83% of patients achieved a CCyR after 3 months, 35% a major MR at one year, and 67% after two years [
27]. On the contrary, 52% of patients had to switch to second generation TKI inhibitors due to treatment failure or AEs in a series of 27 patients treated in Bosnia with three different formulation of generic imatinib upfront [
28]. These studies were performed outside the EU, and different quality controls exist in these countries. Indeed, the level of quality controls used in a country seems more relevant than the origin of the used drug.
Assessing safety and efficacy of generic imatinib formulations is of paramount importance: imatinib mesylate has dramatically changed the prognosis of CML patients and new generic formulations must guarantee similar efficacy and safety profiles. Pharmacoeconomics considerations must also be done to ensure a lower price (approximately 30%) of generic imatinib, especially in countries that do not guarantee free access to this life saving drug. For example, Padula
et al. [
29] estimated that the use of generic imatinib as a first-line drug would be, on average, $91,163 less expensive for patients over 5 years than other therapies such as dasatinib, nilotinib, or branded imatinib in the USA. The authors also argued that the use of generic imatinib would be possible without a clinically meaningful difference in quality-adjusted life-years [
29]. The steep decrease in price will greatly favor a more widespread use of generic imatinib, and better compliance to treatment in those countries in which is not fully reimbursed by local Health Systems [
30].
Finally, our data indicate that generic imatinib does not have deleterious effects on CML control and presents an acceptable safety profile, similar to that of branded imatinib. These data will be useful to clarify doubts and fears among CML patients and doctors about generic safety and effectiveness, provided that strict quality controls be implemented.
Go to :