Abstract
Background
Rare inherited coagulation factor deficiencies constitute an important group of bleeding disorders. A higher frequency of these disorders is seen in areas of high consanguinity. Our aim was to study the prevalence and spectrum of rare inherited bleeding disorders, characterize the severity of the deficiencies, identify different clinical manifestations, and evaluate different treatments provided.
Methods
This cross-sectional study was conducted in the Department of Haematology, Armed Forces Institute of Pathology Rawalpindi, between January 2014 and December 2018. A detailed history was taken, and an examination was performed. The signs and symptoms were noted, and the patients were diagnosed on the basis of a coagulation profile. The disease severity was assessed using factor assays.
Results
Among 2,516 patients with suspected coagulation disorders, 774 (30.8%) had an inherited bleeding disorder. Of the 774 patients, 165 (21.3%) had a rare bleeding disorder; 91 (55.2%) of them were males, and 74 (44.9%) were females, with a male-to-female ratio of 1.21. The median patient age was 9 years 3 months. The most common disorder was factor VII deficiency (46 patients, 27.9%). The most common clinical presentation was bruising in 102 (61.8%) and gum bleeding in 91 (55.2%) patients.
Rare bleeding disorders are a heterogeneous group of inherited coagulation disorders resulting from deficiencies in fibrinogen, prothrombin, factor V, factor VII, factor X, factor XIII, other than von Willebrand disease, factor VIII deficiency, and factor IX deficiency. It also includes some rare combined factor deficiencies such as combined factor V and VIII deficiency [1]. These disorders are usually transmitted in an autosomal recessive manner, apart from fibrinogen and factor XI deficiencies, which are inherited in an autosomal dominant manner. A higher frequency of these disorders is seen in populations in which consanguineous marriages are common [2]. Pakistan is one of the countries where marriages within the same family and within the same tribe are the norm; thus, the incidence of autosomal recessive disorders is high [3, 4].
The clinical spectrum is diverse [5]; while some patients may experience it in the neonatal period, others may present with postoperative or post-traumatic bleeding or may be completely asymptomatic [6]. Bleeding may range from mild occasional epistaxis or gum bleeding to life-threatening bleeding such as intracranial hemorrhage [7]. In some of these rare bleeding disorders, the degree of factor deficiency correlates with the severity of bleeding, whereas in the others, there is a marked phenotype–genotype disparity [8, 9].
These disorders are unique in the sense that they have a low prevalence in the general population and have a highly variable clinical presentation [7]. Thus far, there are no data on the prevalence of these rare bleeding disorders in developing countries including Pakistan. Although the incidence of these disorders is low worldwide, in developing countries, these disorders are underdiagnosed and under-reported. This may be due to the lack of diagnostic facilities for specialized tests including factor assays. Lack of knowledge on the frequency of these disorders in our population causes a lack of public awareness and a low clinical suspicion for these disorders; hence, many of these cases are missed.
Although rare, these disorders may present with significant bleeding; thus, early diagnosis is important to choose the right therapeutic decision [10]. Treatment options vary depending on the type of factor deficiency [11]. While definitive replacement therapy is achieved by factor concentrates, the availability of these factor concentrates in resource-constrained countries is limited, and even when available, the high cost of these factor concentrates is a major concern. For these reasons, clinicians may have to resort to fresh-frozen plasma (FFP) and cryoprecipitate to treat these patients. Establishing a correct diagnosis is of paramount importance to select a treatment, whether it is the specific factor concentrate or plasma product [12].
Pakistan, a developing country with limited resources, has lack of diagnostic facilities. The present study was designed to provide comprehensive data on the frequency of these rare bleeding disorders in this country. We conducted this study to describe the pattern of these bleeding disorders and the clinical presentations of these patients and to study the treatment provided to these patients presenting at our institute. The study findings will be useful to establish a database of these rare bleeding disorders in our population, which will help clinicians in the early diagnosis and to plan treatment accordingly.
This cross-sectional study was conducted at the Armed Forces Institute of Pathology, a tertiary care referral center providing specialized diagnostic facilities for coagulation disorders. The study was approved by the Ethical Review Committee of the Armed Forces Institute of Pathology, Rawalpindi. A total of 2,516 patients were enrolled in the study after obtaining informed consent from the patients, parents, and/or guardians. All patients presenting with a bleeding history or suspicion of an inherited coagulation disorder from January 2014 to December 2018 were included in the study. However, patients diagnosed with platelet function disorders or any acquired coagulation disorder were excluded from the study.
Demographic details were noted. A detailed history including past and present bleeding history, site(s) of bleeding, number of bleeding episodes, family history and consanguinity, treatment history, and history of transfusions was taken. Clinical examination was conducted, especially for any bruising or petechiae. The symptoms and signs were noted. Liver function tests were carried out to rule out any liver pathology affecting the coagulation profile.
Two milliliters of venous blood collected in EDTA was analyzed using Sysmex XE-5000 automated hematology cell counter (Sysmex Corporation, Kobe, Japan) for complete blood counts. Bleeding time was performed by Ivy’s method. For coagulation assays, venous blood was collected in trisodium citrate in a ratio of 9:1. Initially, prothrombin time (PT) and activated partial thromboplastin time (aPTT) were performed on an automated coagulation analyzer CA-1500 (Sysmex, Kobe, Japan). The normal control values were 11 seconds for PT and 25 seconds for aPTT Thrombin time was performed manually using Helena (BD Biosciences, San Jose, CA, USA) thrombin time reagent. Urea clot solubility test using 5 M urea was performed to assess factor XIII. All abnormal results were repeated manually using Helena PT and aPTT reagents, followed by inhibitor screen by mixing 1:1 with normal plasma. Mixing studies using in-house- prepared adsorbed plasma and aged serum were then performed. Based on the results, specific factor assays were performed on the automated hematology analyzer CA-1500. PT-based factor assay was performed for factor V, VII, and X, while aPTT-based factor assays were performed for factor VIII, IX, XI, and XII using Siemens factor-deficient plasma. Reference values for factor assays were 70–150% for aPTT-based assays as per the manufacturer’s instructions. All patients with factor V deficiency were also checked for combined factor V and factor VIII deficiency by factor VIII assay. For suspected fibrinogen deficiencies, quantitative determination of fibrinogen based on the principle of Clauss assay was carried out for fibrinogen estimation on the automated coagulation analyzer CA-1500. Normal reference values for fibrinogen were 1.8–3.5 g/L as provided by the manufacturer. Patients with vitamin K deficiency bleeding underwent a trial of vitamin K, and their samples were repeated and analyzed. The tests for each sample were duplicated to confirm factor deficiency.
The data were entered and analyzed using IBM SPSS software (Statistical Package for Social Sciences, version 20, IBM, Armonk, NY, USA). Categorical variables were expressed as frequency and percentage, whereas quantitative variables were expressed as mean and standard deviation (mean±SD). The baseline characteristics were compared using chi-square test. All P-values were two-sided. P<0.05 was considered to indicate statistical significance.
A total of 2,516 patients with suspected coagulation disorders were enrolled in our study. Among them, 774 (30.8%) were diagnosed with an inherited bleeding disorder. Among the 774 patients, 165 (21.3%) were diagnosed to have a rare bleeding disorder, while 264 (34.1%) were diagnosed with Hemophilia A, 63 (8.1%) with Hemophilia B, and 156 (20.2%) with von Willebrand disease (Table 1).
Of the 165 patients diagnosed with rare bleeding disorders, 91 (55.2%) were males, while 74 (44.9%) were females, with a male-to-female ratio of 1.2:1. The median age of these patients was 9 years 3 months, with a range of 4 months to 42 years, at the time of presentation to our tertiary care center. The most common deficiency was factor VII deficiency in 46 (27.9%) patients, followed by fibrinogen deficiency in 35 (21.2%) patients, factor V deficiency in 27 (16.4%) patients, factor XIII deficiency in 19 (11.5%) patients, factor X deficiency in 19 (11.5%) patients, factor XI deficiency in 14 (8.5%) patients, and factor XII deficiency in 2 (1.2%) patients, while no patient had factor II deficiency (Table 2). There were three (1.8%) patients with combined factor V and VIII deficiency. Female predominance was seen in fibrinogen deficiency and factor XIII deficiency.
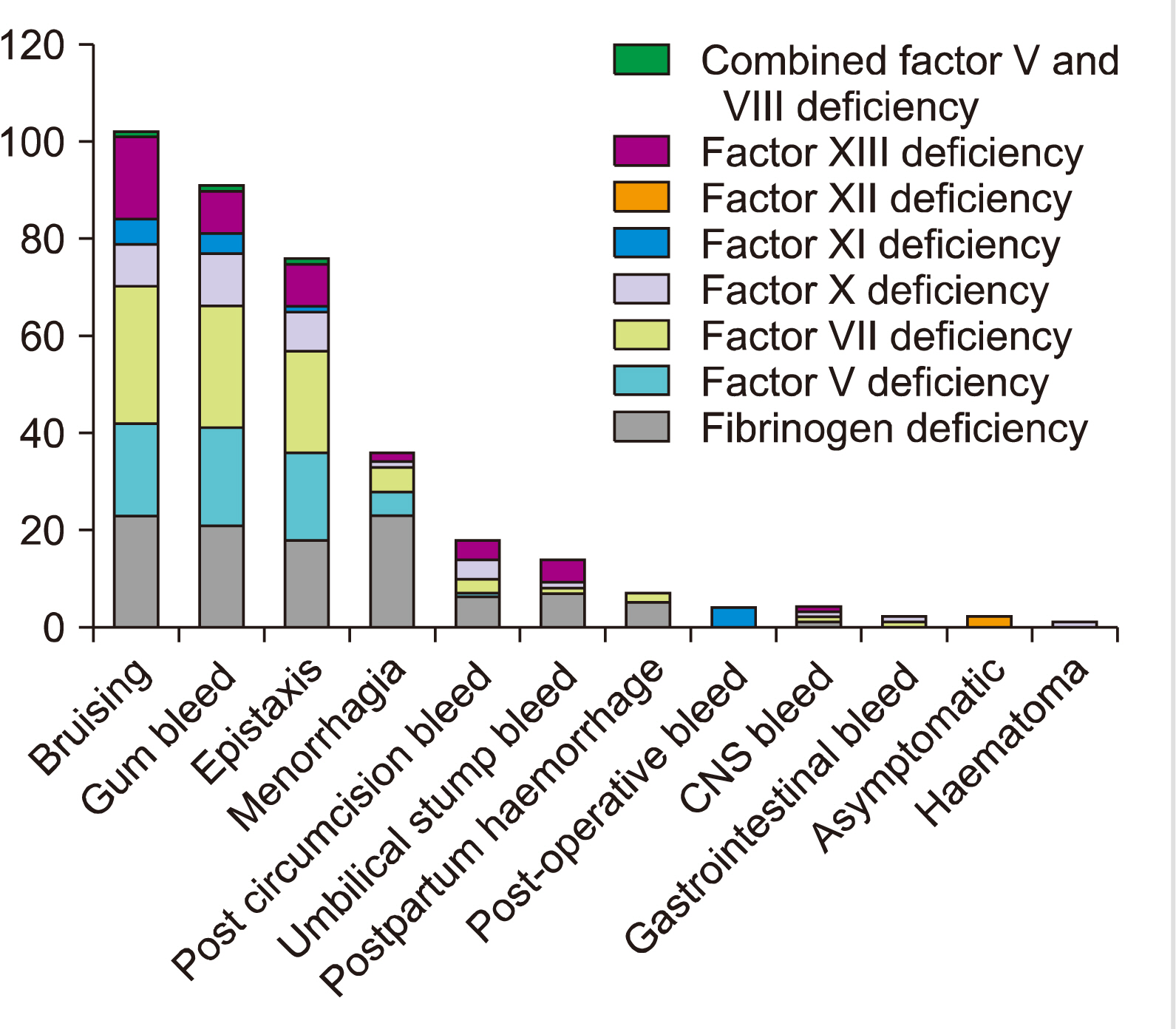
Consanguinity was observed in 134 (81.2%) patients. Nineteen (11.5%) patients diagnosed with rare bleeding disorders had a family history. The median age at first bleeding was 1 year 6 months, with a range of 3 days to 13 years. The most common clinical presentation was bruising in 102 (61.8%) and gum bleeding in 91 (55.2%) patients, followed by epistaxis in 76 (46.1%) and menorrhagia in 36 (21.8%) patients (Fig. 1). In our cohort, the most severe symptoms of bleeding were observed in factor X deficiency, followed by factor VII deficiency and factor XIII deficiency. Intracranial bleeding was seen in two patients with factor X deficiency, one patient with factor VII and the other with factor XIII deficiency. Umbilical stump bleeding was a prominent presenting feature in patients with factor XIII and fibrinogen deficiencies. Menorrhagia was the predominant presentation in females, particularly in patients with fibrinogen, factor VII, and factor X deficiencies.
On follow-up, bleeding episodes were controlled only by local measures in 20 (12.1%) patients, and antifibrinolytics were the mainstay treatment in 49 (29.7%) patients. Eighty- eight (53.3%) patients required transfusions for their bleeding. Of them, 41 (24.8%) had received FFPs at least once, whereas 36 (21.8%) received FFP transfusions intermittently. Eleven (6.7%) patients received cryoprecipitate. Five (3.0%) patients received recombinant FVII (rFVIIa): three females required it at the time of delivery/cesarean section, one required it for postpartum hemorrhage, and a young boy needed it at the time of laparotomy. Three (1.8%) patients required no treatment during the follow-up period.
Knowledge of the prevalence of these disorders, clinical presentations, and management strategies has been established, and advanced treatment options are emerging in developed countries. However, in developing resource-constrained countries, delay in diagnosis and sub-optimal availability of therapeutic modalities have been major challenges in the management of patients with rare bleeding disorders. The biological heterogeneity and geographical variations in disease presentation and severity warrant the formulation of a registry for our population. Establishing a database for our population is essential for the timely diagnosis of these patients and to establish clinical treatment guidelines appropriate for our set-up and resources.
In our study, the frequency of rare bleeding disorders was 21.3% in patients diagnosed with an inherited bleeding disorder. Mansouritorghabeh et al. [13] reported a frequency of 15.6% in the Iranian population. The most common rare bleeding disorder in our population was factor VII deficiency (27.9%), followed by fibrinogen deficiency (21.2%). A study conducted in Fars Hemophilia Center, affiliated with the Shiraz University of Medical Sciences, Iran, reported factor VII deficiency as the most frequent rare bleeding disorder, followed by factor X deficiency [14]. Sharma et al. [15] reported factor X deficiency as the most common rare bleeding disorders, followed by factor XIII and factor VII deficiencies. A retrospective analysis of 156 patients in Turkey revealed factor VII and factor V deficiencies as the most common rare bleeding disorders [16].
Autosomal recessive disorders are more commonly seen in regions with a high rate of consanguinity; this necessitates the establishment of diagnostic facilities to ensure prompt diagnosis and accurate treatment [17]. Among patients with rare bleeding disorders, consanguinity was found in 47.2% of the Turkish population and in 81.2% of our patients [18]. Mucocutaneous bleeding was the most common complaint in our population. Sharma et al. [15] reported similar findings. In our cohort, clinically significant bleeding was observed in patients having factor X deficiency. Intracranial bleeding was seen in two patients with factor X deficiency, one patient with factor VII and the other with factor XIII deficiency. However, an Indian study reported factor XIII deficiency as the most common cause of intracranial bleeding [15]. A comparison of the frequencies of different rare coagulation factor deficiencies between our data and international data is shown in Fig. 2 [19].
This is one of the largest reported studies on rare bleeding disorders from our region. We have tried to comprehensively cover the demographics, clinical presentations, disease severity, and different treatments received. However, our study included only patients presenting to the hospital or referred to us. A large proportion of the population living in rural areas who do not have access to tertiary care diagnostic facilities remain undiagnosed. Thus, the frequency of these bleeding disorders in our population may be much higher than that reported. A limitation of this study is that the diagnosis of rare blood disease (RBD) was based on coagulation test results only, without molecular genetic studies, as Pakistan is a resource-constrained country and the facility used for the molecular diagnosis of RBDs is not available in our country. However, future studies on the molecular basis of RBDs are required to confirm the genetic nature of the deficiency in the Pakistani population. Knowledge of the incidence of these disorders will help to establish facilities for early detection and appropriate management, thus preventing life-threatening bleeding and mortality.
REFERENCES
1. Palla R, Peyvandi F, Shapiro AD. 2015; Rare bleeding disorders: diagnosis and treatment. Blood. 125:2052–61. DOI: 10.1182/blood-2014-08-532820. PMID: 25712993.

2. Naderi M, Tabibian S, Hosseini MS, et al. 2014; Rare bleeding disorders: a narrative review of epidemiology, molecular and clinical presentations, diagnosis and treatment. JPR. 2:31–46.
3. Peyvandi F, Palla R, Menegatti M, et al. 2012; Coagulation factor activity and clinical bleeding severity in rare bleeding disorders: results from the European Network of Rare Bleeding Disorders. J Thromb Haemost. 10:615–21. DOI: 10.1111/j.1538-7836.2012.04653.x. PMID: 22321862.

4. Dorgalaleh A, Alavi SE, Tabibian S, et al. 2017; Diagnosis, clinical manifestations and management of rare bleeding disorders in Iran. Hematology. 22:224–30. DOI: 10.1080/10245332.2016.1263007. PMID: 27894217.

5. Öner N, Gürsel T, Kaya Z, et al. 2020; Inherited coagulation disorders in Turkish children: a retrospective, single-center cohort study. Transfus Apher Sci. 59:102728. DOI: 10.1016/j.transci.2020.102728. PMID: 31980335.

6. de Moerloose P, Schved JF, Nugent D. 2016; Rare coagulation disorders: fibrinogen, factor VII and factor XIII. Haemophilia. 22(Suppl 5):61–5. DOI: 10.1111/hae.12965. PMID: 27405678.

7. Acharya SS, Coughlin A, Dimichele DM. North American Rare Bleeding Disorder Study Group. 2004; Rare bleeding disorder registry: deficiencies of factors II, V, VII, X, XIII, fibrinogen and dysfibrinogenemias. J Thromb Haemost. 2:248–56. DOI: 10.1111/j.1538-7836.2003.t01-1-00553.x. PMID: 14995986.

8. Mumford AD, Ackroyd S, Alikhan R, et al. 2014; Guideline for the diagnosis and management of the rare coagulation disorders: a United Kingdom Haemophilia Centre Doctors' Organization guideline on behalf of the British Committee for Standards in Haematology. Br J Haematol. 167:304–26. DOI: 10.1111/bjh.13058.
9. Peyvandi F, Di Michele D, Bolton-Maggs PH, et al. 2012; Classification of rare bleeding disorders (RBDs) based on the association between coagulant factor activity and clinical bleeding severity. J Thromb Haemost. 10:1938–43. DOI: 10.1111/j.1538-7836.2012.04844.x. PMID: 22943259.

10. Colvin BT, Astermark J, Fischer K, et al. 2008; European principles of haemophilia care. Haemophilia. 14:361–74. DOI: 10.1111/j.1365-2516.2007.01625.x. PMID: 18248408.

11. Jain S, Acharya SS. 2018; Management of rare coagulation disorders in 2018. Transfus Apher Sci. 57:705–12. DOI: 10.1016/j.transci.2018.10.009. PMID: 30392819.

12. Peyvandi F, Garagiola I, Biguzzi E. 2016; Advances in the treatment of bleeding disorders. J Thromb Haemost. 14:2095–106. DOI: 10.1111/jth.13491. PMID: 27590165.

13. Mansouritorghabeh H, Manavifar L, Banihashem A, et al. 2013; An investigation of the spectrum of common and rare inherited coagulation disorders in north-eastern Iran. Blood Transfus. 11:233–40. DOI: 10.2450/2012.0023-12. PMID: 23114518. PMCID: PMC3626474.
14. Farjami A, Haghpanah S, Arasteh P, et al. 2017; Epidemiology of hereditary coagulation bleeding disorders: a 15-year experience from Southern Iran. Hospital Practices and Research. 2:113–7. DOI: 10.15171/hpr.2017.27.

15. Sharma SK, Kumar S, Seth T, et al. 2012; Clinical profile of patients with rare inherited coagulation disorders: a retrospective analysis of 67 patients from northern India. Mediterr J Hematol Infect Dis. 4:e2012057. DOI: 10.4084/mjhid.2012.057. PMID: 23170186. PMCID: PMC3499996.

16. Fışgın T, Balkan C, Celkan T, et al. 2012; Rare coagulation disorders: a retrospective analysis of 156 patients in Turkey. Turk J Haematol. 29:48–54. DOI: 10.5505/tjh.2012.02418. PMID: 24744623. PMCID: PMC3986768.
17. Jaouad IC, Elalaoui SC, Sbiti A, Elkerh F, Belmahi L, Sefiani A. 2009; Consanguineous marriages in Morocco and the consequence for the incidence of autosomal recessive disorders. J Biosoc Sci. 41:575–81. DOI: 10.1017/S0021932009003393. PMID: 19433002.

18. Şalcıoğlu Z, Bayram C, Şen H, et al. 2018; Congenital factor deficiencies in children: a report of a single-center experience. Clin Appl Thromb Hemost. 24:901–7. DOI: 10.1177/1076029617731596. PMID: 29050499. PMCID: PMC6714728.

19. Peyvandi F, Kaufman RJ, Seligsohn U, et al. 2006; Rare bleeding disorders. Haemophilia. 12(Suppl 3):137–42. DOI: 10.1111/j.1365-2516.2006.01271.x. PMID: 30392819.

Fig. 2
Comparison of the frequency of rare coagulation factor defici-encies between our data and international data [19].
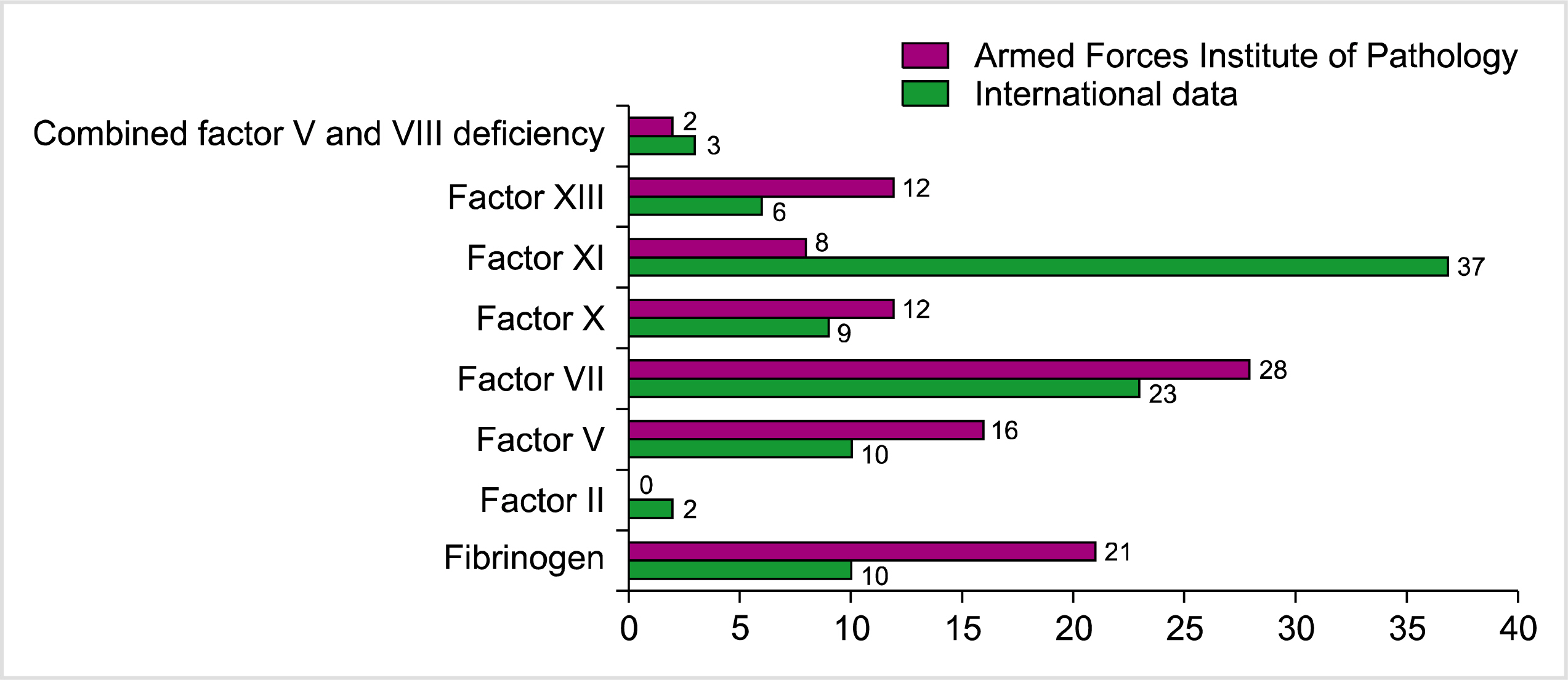
Table 1
Patients presenting with bleeding disorders.
Table 2
Frequency distribution and features of patients with rare bleeding disorders.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download