TO THE EDITOR: Pulmonary medicine consultation was sought for a 65-year-old man referred from an outside hospital with complaints of breathlessness, mediastinal lymphadenopathy, and intermittent low-grade fever accompanied by worsening anemia and low total leukocyte count. The patient had a history of one month of first-line anti-tuberculous therapy (ATT) based on a provisional diagnosis of pulmonary tuberculosis. His chest radiography and thoracic contrast-enhanced computerized tomography (CECT) scan revealed no pulmonary lesion but anterior mediastinal lymphadenopathy only. Physical examination revealed only pallor, without palpable lymphadenopathy or hepatosplenomegaly. A complete radiological examination performed outside did not reveal any abnormalities other than mediastinal lymphadenopathy, which was also corroborated by positron emission tomography (PET)-CT scan.
Routine laboratory investigation revealed normocytic normochromic anemia [hemoglobin (Hb), 60 g/L; mean corpuscular volume (MCV), 88 fL], leukopenia (leucocyte count, 2×109/L) with relative lymphocytic prominence (30% neutrophil, 60% lymphocytes, 4% eosinophils, 6% monocytes), and adequate platelet count (350×109/L). Peripheral smears revealed scattered suspicious lymphoid cells constituting 10% of all lymphoid cells (Fig. 1). A review of bone marrow aspiration (BMA) slides performed at the referring hospital for evaluation of his cytopenia(s) revealed hemodilution with suppressed trilineage hematopoiesis and no abnormal cells, indicating a suspicion of marrow hypoplasia. Trephine core sections showed a variably cellular marrow with suppressed trilineage hematopoiesis with near-total replacement by diffuse sheets of a monotonous population of small to intermediate-sized atypical lymphoid cells with round to mildly irregular nuclear contours, dense chromatin, and inconspicuous nucleoli. In places, these cells exhibited perinuclear clear zones with a fried egg appearance. There was no evidence of increased mitosis, granuloma, necrosis, or any large cell transformation. Reticulin staining showed myelofibrosis (MF) grade 0–1 condensation [World Health Organization (WHO) classification]. Immunohistochemical investigation revealed lymphoid cells with strong and diffuse positivity for CD20, CD11c, and BCl2; weak immunopositivity for CD10 and kappa light chain; and negativity for CD3, CD5, CD23, BCl6, CD138, cyclin D1, CD30, ALK1, CD34, CD117, and lambda light chain, consistent with a low-grade B cell lymphoproliferative neoplasm (LPN) of hairy cell phenotype (Fig. 2). The results of serum immunofixation electrophoresis and Coomb test were within normal limits. A repeat BMA sample was subjected to multiparametric flow cytometric immunophenotyping, which showed bright CD200, CD25, CD11c, CD19, and CD20 expression, consistent with hairy cell leukemia (HCL). Dim to moderate CD23 expression was also noted in a subset of these gated lymphoid cells (Fig. 3). The patient was referred to a specialized tertiary care cancer institute for further management; he was started on cladribine treatment and achieved a complete response and is presently under follow-up. Molecular testing for BRAF was not done as per the clinician's decision.
HCL is a rare, clinically indolent, mature small B cell lymphoproliferative neoplasm (LPN) that accounts for 2% of all lymphoid leukemias. It is characterized by the proliferation of small lymphoid cells with abundant cytoplasm with circumferential fine hairy projections involving the peripheral blood, bone marrow, and expanding splenic red pulp. The hallmark features are marked splenomegaly, peripheral blood cytopenia(s), monocytopenia, and circulating CD19+, bright CD20+/CD22+/CD200+, CD25+, CD103+, and CD11c+ hairy cells demonstrated classically by flowcytometric analysis. Annexin A1 is the most specific marker for HCL, which helps in differentiating HCL mimics such as variant HCL (vHCL) and splenic marginal zone lymphoma, which are both annexin A1-negative [1].
Atypical or unusual presentation in HCL (aHCL) is uncommonly reported in the literature (1990 to 2018), which may pose a significant diagnostic challenge in routine practice (Table 1) [2-10]. Splenohepatomegaly is conspicuously absent which, in the presence of peripheral blood cytopenia(s), resembled hypoplastic anemias. Significant lymphadenopathy; skeletal manifestation in the form of arthralgia, multifocal lytic to sclerotic bone lesions; neurological manifestations; isolated epidural, skull base, or breast lesions have dominated the clinical presentations in other reports [2,4-9]. HCs were conspicuously absent to very sparse; both in peripheral blood smear (PBS) and scant bone marrow aspirate (BMA) smears requiring tartrate-resistant acid phosphatase (TRAP) cytochemistry. The trephine sections resembled hypoplastic marrow with atypical lymphoid cells forming discrete or nodular aggregates unlike those of classical HCL. Diffuse infiltration by low-grade LPN gave a typical ‘fried egg’ appearance in others. Others showed classic histomorphology in extramedullary sites without evidence of marrow involvement. Flowcytometric and/or IHC analysis demonstrated classical HCL phenotypes with bright CD200 coexpression in all cases reported in the literature [11, 12]. Immunophenotypic aberrancies such as heterogenous CD10 and CD23 coexpression are reported in up to 15% cases; whereas CD5+ and CD103/CD25- were sporadically reported and were not associated with any specific laboratory abnormalities. Atypical presentations or immunophenotypic aberrancies did not alter the favorable response to purine analogs such as cladribine [7, 11].
In summary, unusual or atypical clinical presentation is not uncommon in HCL. Furthermore, rare immunophenotypic aberrancies in HCL should be considered when interpreting flow cytometric/immunohistochemical patterns in such cases to avoid misdiagnosis.
REFERENCES
1. Foucar K, Falini B, Stein H. Swerdlow SH, Campo E, Harris NL, editors. 2017. Hairy cell leukemia. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. IARC Press;Lyon, France:
2. Moustafa A, Shwaylia HM, Aldapt M, Yassin MA. 2019; Outcome of atypical presentations of hairy cell leukemia. Blood (ASH Annual Meeting Abstracts). 134(Suppl):5267. DOI: 10.1182/blood-2019-121826.

3. Rahman K, Kumari S, Singh MK, et al. 2018; Atypical presentation of hairy cell leukemia: significance of CD200 on flow cytometry. J Cancer Res Ther. 14:1130–4. DOI: 10.4103/0973-1482.188432. PMID: 30197362.

4. Alfaraj M, Alsaeed H. 2017; Hairy cell leukemia: a case report of atypical presentation without splenomegaly. Blood Res. 52:139–41. DOI: 10.5045/br.2017.52.2.139. PMID: 28698853. PMCID: PMC5503894.

5. Chai KL, Morris T, Bazargan A. 2017; A case report of hairy cell leukemia: an unusual presentation. Ann Clin Case Rep. 2:1412.
6. Yonal-Hindilerden I, Hindilerden F, Bulut-Dereli S, Yıldız E, Dogan IO, Nalcaci M. 2015; Hairy cell leukemia presenting with isolated skeletal involvement successfully treated by radiation therapy and cladribine: a case report and review of the literature. Case Rep Hematol. 2015:803921. DOI: 10.1155/2015/803921. PMID: 26788382. PMCID: PMC4695657.

7. Gupta AK, Sachdeva MU, Ahluwalia J, et al. 2015; Haematological profile of 21 patients with hairy cell leukaemia in a tertiary care centre of north India. Indian J Med Res. 142:426–9. DOI: 10.4103/0971-5916.169204. PMID: 26609034. PMCID: PMC4683827.

8. Pilichowska M, Shariftabrizi A, Mukand-Cerro I, Miller K. 2014; Primary hairy cell leukemia/lymphoma of the breast: a case report and review of the literature. Case Rep Pathol. 2014:497027. DOI: 10.1155/2014/497027. PMID: 25133005. PMCID: PMC4123510.

9. Venkatesan S, Purohit A, Aggarwal M, et al. 2014; Unusual presentation of hairy cell leukemia: a case series of four clinically unsuspected cases. Indian J Hematol Blood Transfus. 30(Suppl 1):413–7. DOI: 10.1007/s12288-014-0442-9. PMID: 25332634. PMCID: PMC4192250.

10. Rosen DS, Smith S, Gurbuxani S, Yamini B. 2008; Extranodal hairy cell leukemia presenting in the lumbar spine. J Neurosurg Spine. 9:374–6. DOI: 10.3171/SPI.2008.9.10.374. PMID: 18939925.

11. Chen YH, Tallman MS, Goolsby C, Peterson L. 2006; Immunophenotypic variations in hairy cell leukemia. Am J Clin Pathol. 125:251–9. DOI: 10.1309/PMQXVY619Q8Y43AR. PMID: 16393677.

12. Sandes AF, de Lourdes Chauffaille M, Oliveira CR, et al. 2014; CD200 has an important role in the differential diagnosis of mature B-cell neoplasms by multiparameter flow cytometry. Cytometry B Clin Cytom. 86:98–105. DOI: 10.1002/cytob.21128.

Fig. 1
Spectrum of circulating lymphoid cells in the peripheral blood in this case. Note the absence of characteristic circumferential hair-like projections in most cells (Leishman Giemsa stain, ×400).
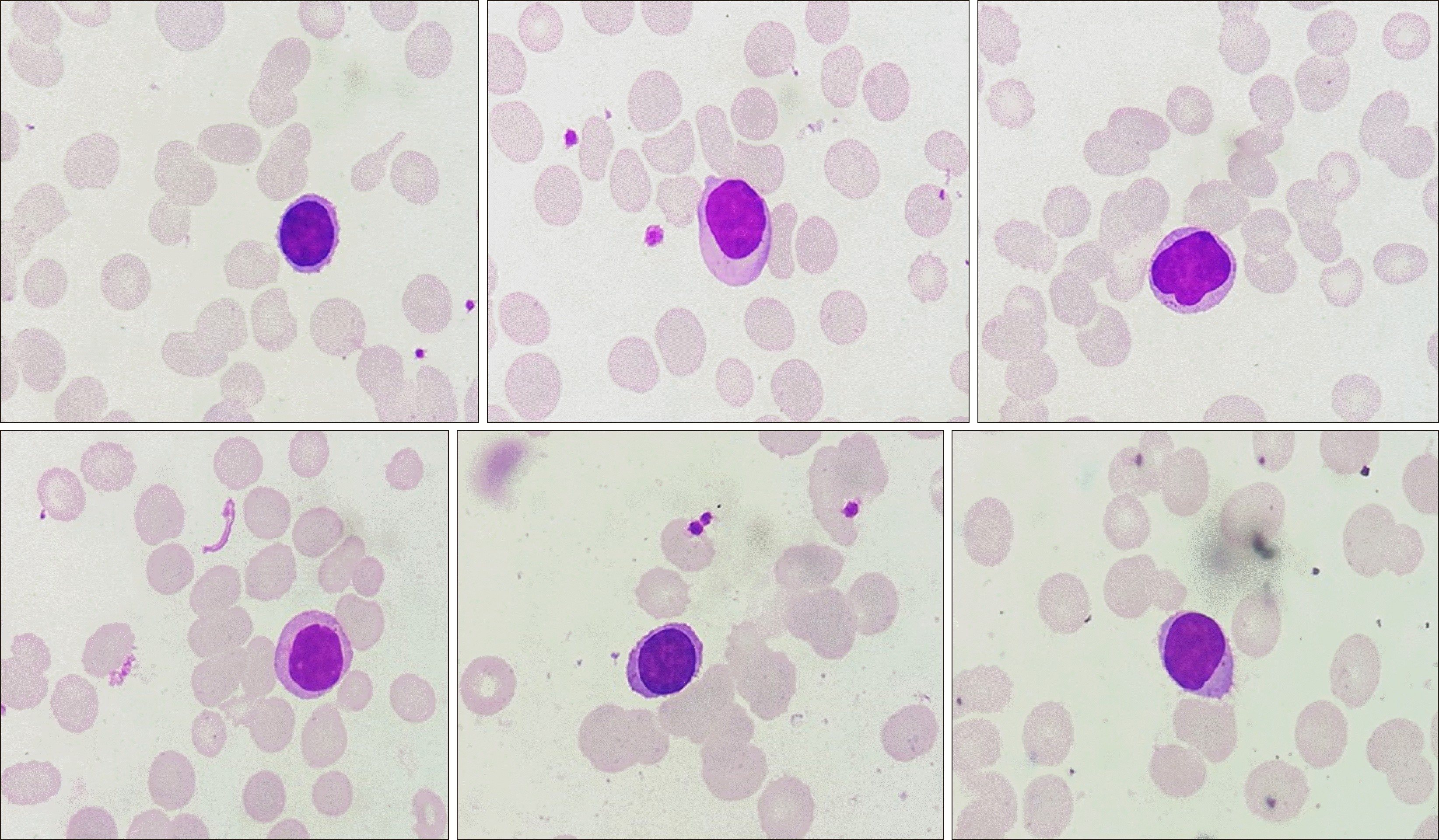
Fig. 2
Trephine marrow sections from the case demonstrating diffuse sheets of small mature-looking lymphoid cells with round to mildly irregular nuclear outlines and abundant clear cytoplasm with a ‘fried egg’ pattern classical of hairy cell leukemia (A, Hematoxylin and eosin, ×400). On immunohistochemistry, these lymphoid cells show strong diffuse positivity for CD20 (B), CD11c (not shown), and CD10 (C, peroxidase-antiperoxidase, ×400).
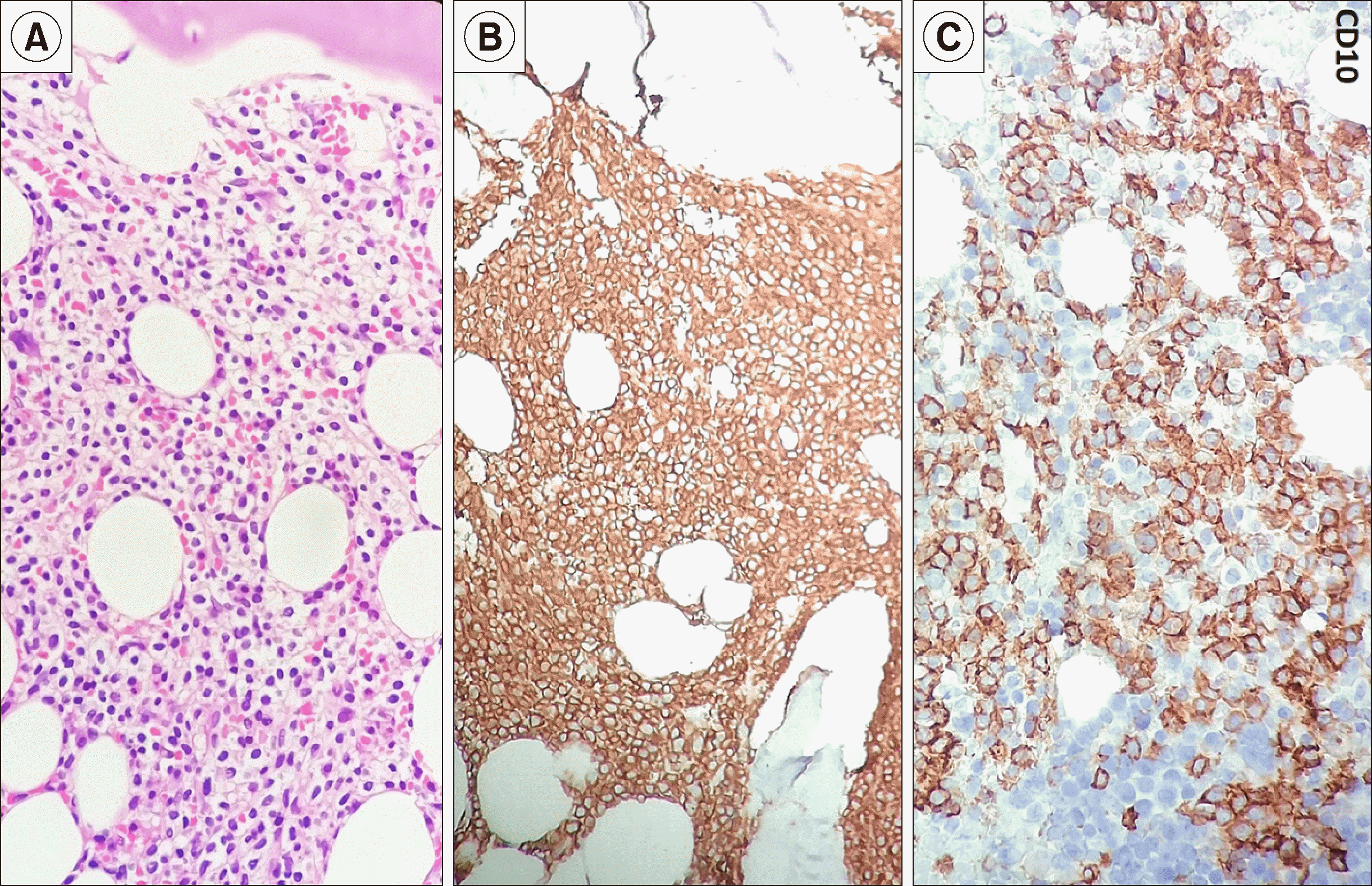
Fig. 3
Flow cytometric immunophenotyping plots with the population of interest (B cells) gated in red on CD19 versus side-scattered light (SSC). The B cells are positive for CD11c (bright), CD25 (bright), CD200, CD103, and CD123 diagnostic of hairy cell leukemia. Note the dim to moderate CD23 positivity in the CD19+ lymphoid cells as previously reported in the literature.
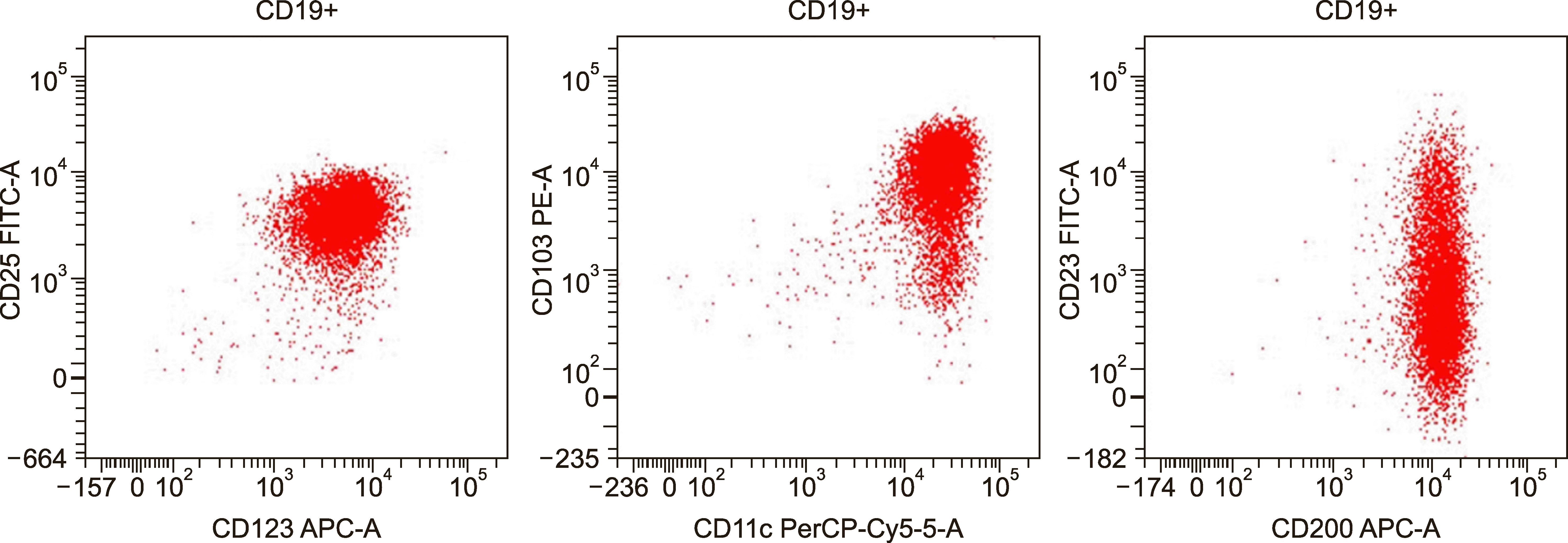
Table 1
Atypical presentations of hairy cell leukemia: a comprehensive literature review.
| Ref. | Age (yr)/sex | Presentation | Hairy cells | Immunophenotyping (FC/IHC) | Therapy; outcome | Molecular studies | ||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| PBS | BMA | BMBx/tissue Bx | ||||||
| [3] | 52/M | Pancytopenia (? aplastic); no spleen | -/+ | Scant, -/+ | Hypoplastic atypical lymphoid nodule | CD200/CD11c/CD25/CD20/kappa: + | Cladribine; CR | BRAF+ |
| [3] | 56/M | Pancytopenia (? aplastic)no spleen | - | - | Hypoplastic focal fried egg | CD20/annexin A1; + | Cladribine; CR | Not tested |
| [3] | 48/M | Lymphocytosis; massive spleen, multiple LNs | -/+ | -/+ | LPN; fried egg | CD200/CD11c/CD25/CD20/kappa; + | Supportive | Not tested |
| [3] | 55/F | Pancytopenia (? aplastic);no spleen | - | ?/+ | Hypoplastic, atypical lymphoid nodule | CD200/CD11c/CD25/CD20/kappa; + | Cladribine; CR | BRAF+ |
| [4] | 43/F | Arthalgia pancytopenia, no spleen | -/+ | -/+ | Fried egg | CD200/CD11c/CD25/CD20/FMC7/HLA-DR; + | Cladribine; not described | Not tested |
| [5] | 68/M | Lesion in skull base, bones, pleura; low platelet; no spleen mediastinal LN | - | -/+ | LPN (tissue and BM) | Cyclin D1+, CD 19/CD 20; +, CD5/CD23/CD10; -, CD 11c/25/103; + | Cladribine; rituximab; CR | t (11; 14); - BRAF- |
| [6] | 55/M | Lytic to sclerotic bone lesion; rt femur, no spleen | - | - | LPN (bone biopsy) fried egg BM; no abnormality | TRAP/annexin A1/CD20/CD11c; +, FC: no abnormality | RT+ Cladribine, CR | BRAF+ |
| [8] | 44/F | Breast lesion; no spleen | -/+ | Dry tap | <10% atypical lymphoid cells, MF 2 | Breast: CD20/PAX5/Cyclin D1 (variable+)/annexin A1/TRAP;+ CD25; weak+ BM-FC: + (kappa) | Not described | t (11; 14); - BRAF+ |
| [9] | 69/M | Pancytopenia(? aplastic) no spleen | - | -/+ | Hypoplastic, atypical lymphoid nodule, fried egg | IHC: DBA44/annexin A1; + FC: hairy cell leukemia | Cladribine; CR | Not tested |
| [9] | 50/M | Weakness, weight loss, bicytopenia; no spleen | -/+ | -/+ | LPN; fried egg | TRAP (PBS); + FC; hairy cell leukemia | Cladribine; CR | Not tested |
| [9] | 43/M | Recurrent URTI, low platelet; no spleen | -/+ | -/+ (TRAP +ve) | LPN; fried egg | CD200/CD11c/CD25/CD20/kappa; + | Supportive | Not tested |
| [9] | 67/M | Pancytopenia (? aplastic/MDS) no spleen | 10% | -/+ (TRAP +ve) | Hypoplastic; atypical lymphoid nodule,fried egg, MF 1 to 2 | PB-FC: CD 19/CD 11c/CD 25/CD 103; + | Cladribine; CR | Not tested |
| [10] | 54/M | LS epidural mass with radiculopathy, multifocal bone lesions; no spleen | - | Dry tap | LPN fried egg (tissue Bx+BM) | Bx: CD20/TRAP/CD25; + BM-FC: CD19/CD11c/CD103/CD25; + | Cladribine; CR | Not tested |
| Present case | 63/M | Breathlessness; mediastinal LN, leukopenia; no spleen | -/+ | -/+ | LPN; Fried egg | IHC; CD 20/CD 10/CD 11c; +, FC; CD200/CD19/CD11c/CD103/CD 10/CD25; +CD 23; dim to moderate+ | Cladribine, CR | Not tested |
Abbreviations: +, positive; -, negative; -/+, occasional to very few; ?/+, suspicious; BMA, bone marrow aspiration; BMBx, bone marrow trephine biopsy; BRAF, BRAF V600E mutation analysis; CD200, bright positivity in flow cytometry; CR, complete response; F, female; FC, flow cytometry; IHC, immunohistochemistry; LN, lymph nodes; LPN, low grade lymphoproliferative neoplasm; LS, lumbosacral; M, male; MDS, myelodysplastic syndrome; MF, myelofibrosis grade as per WHO; PB, peripheral blood; PBS, peripheral blood smear; RT, radiotherapy; URTI, upper respiratory tract infection.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download