INTRODUCTION
MATERIALS AND METHODS
Donor lymphocytes and recipient sera
Table 1
| Recipient/donor | Parity/relation | N | Sex (M:F) | Mean age, yr (range) | |
|---|---|---|---|---|---|
| Sensitization pairsa) | Recipients | Multipara | 143 | 0:143 | 54 (34–80) |
| Previous sensitizers | Daughter | 29 | 0:29 | 31 (16–46) | |
| Son | 39 | 39:0 | 27 (14–44) | ||
| Husband | 75 | 75:0 | 56 (33–80) | ||
| Total | 143 | 114:29 | 44 (14–80) | ||
| Non-sensitization pairs | Recipients | 6 | 2:4 | 45 (28–56) | |
| Non-sensitizer | 6 | 3:3 | 44 (34–55) | ||
Laboratory procedures
Conventional FCXM
 | Fig. 1Data analysis of FCXM. T cells were gated using lymphgate R1 and T cell gate R2. B cells were gated using lymphgate R1 and B cell gate R3. On the anti-IgG FITC histogram of the gated T cells or B cells, the geometric mean of the peak within the marker M1 was obtained and used to calculate the test/control MFI ratio.
Abbreviations: AB, group AB serum from healthy individuals; DC, donor cells; FITC, fluorescein isothiocyanate; FSC, forward scatter; MFI, mean fluorescence intensity; PE, phycoerythrin; PerCP, peridinin chlorophyll protein complex; RS, recipient serum; SSC, side scatter.
|
Inhibition FCXM
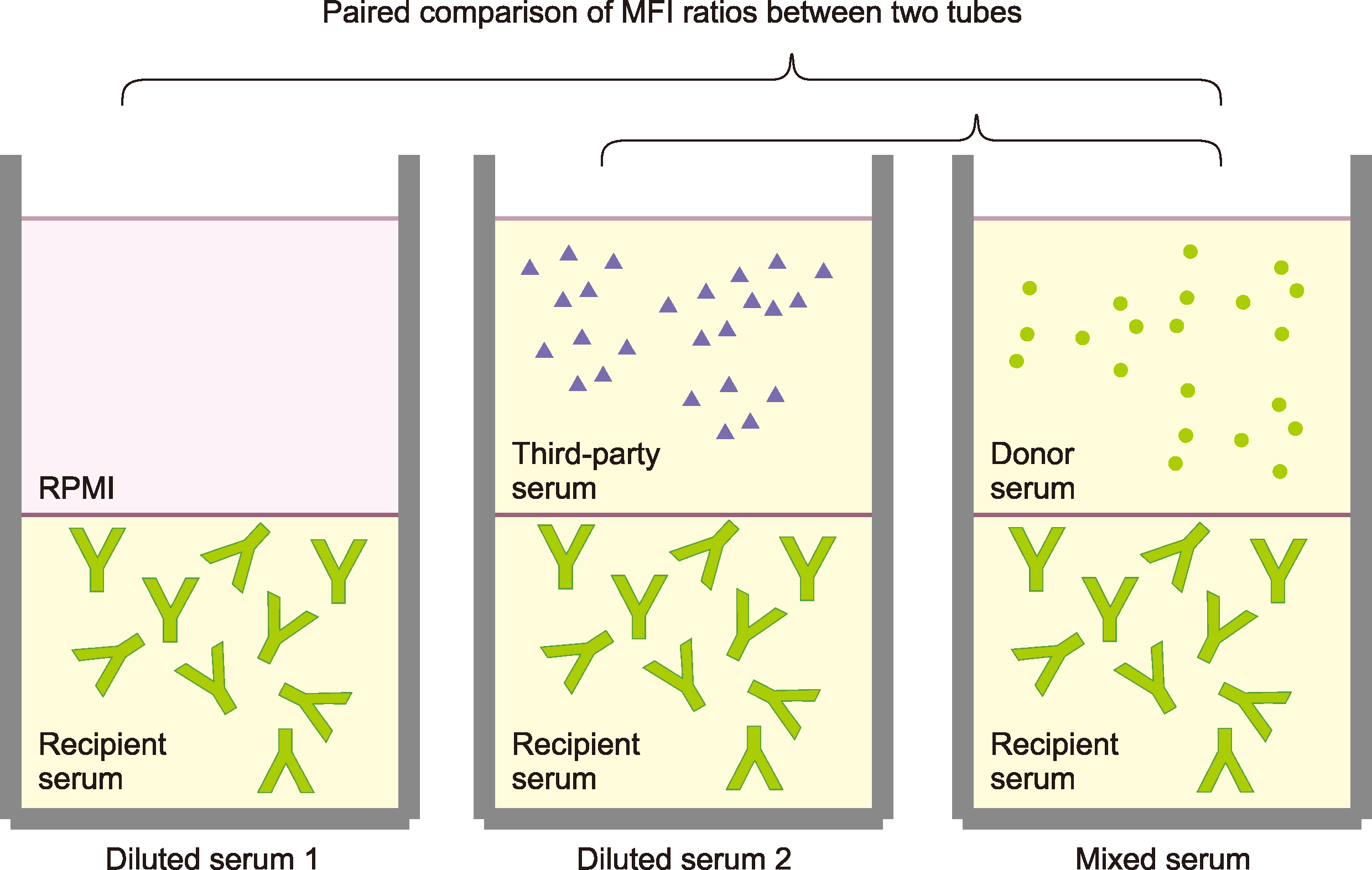 | Fig. 2Preparations of “diluted” or “mixed” serum and paired comparisons among them. Recipient serum was mixed with an equal volume of donor serum (mixed serum) and incubated at 25°C for 30 min. For comparison with mixed serum, the recipient serum was diluted with an equal volume of diluent, either RPMI or third-party serum (diluted serum). The green symbols resembling an uppercase letter Y represent antibodies, and the small round or triangular symbols represent sHLA. In the mixed serum, the sHLA (green round dots) from a donor (previous sensitizer) can neutralize donor-specific antibodies (green “Y”) in recipient serum. |
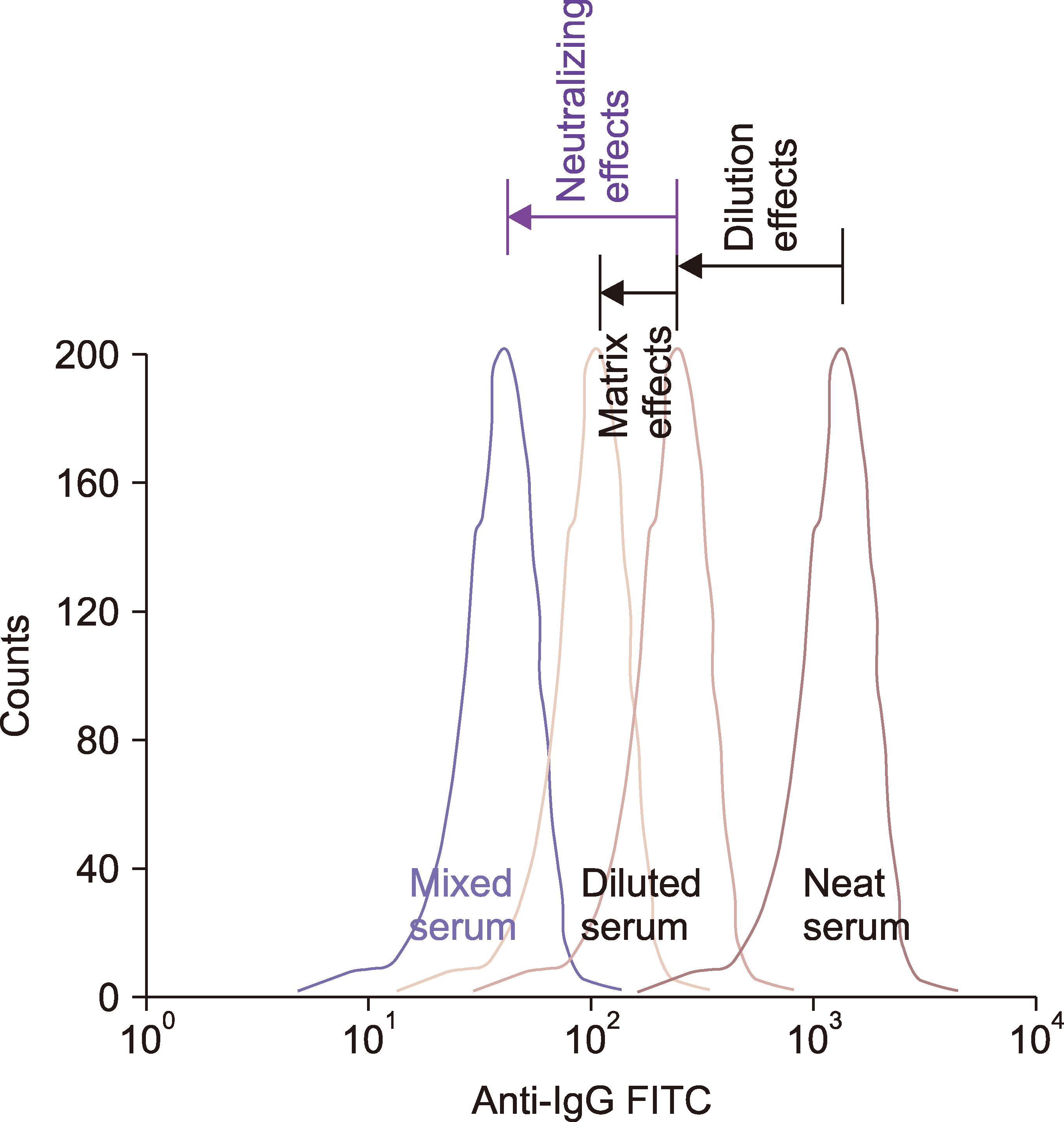 | Fig. 3Representative diagram of neutralizing effects superior to matrix effects on the anti-IgG histogram of gated T cells in FCXM. T cell peaks are shown for mixed serum (donor+recipient), diluted serum (with either RPMI or third-party group AB serum), and neat serum. The peak for the neat serum in conventional FCXM appears in the right-most position, reflecting its original DSA level. In the diluted serum, the dilution and matrix effects are owing to the decreased DSA and immunoglobulin concentrations, respectively. Neutralizing effects in the mixed serum are evident only when they surpass the matrix effects in the corresponding diluted serum (MFI, diluted>mixed). In contrast, when there are no neutralizing effects, only matrix effects are apparent (MFI, mixed>diluted).
Abbreviations: DSA, donor-specific HLA alloantibody; FITC, fluorescein isothiocyanate; MFI, mean fluorescence intensity; sHLA, soluble human leukocyte antigen.
|
Statistical analysis
RESULTS
Conventional FCXM
Table 2
Neutralizing effects of donor serum
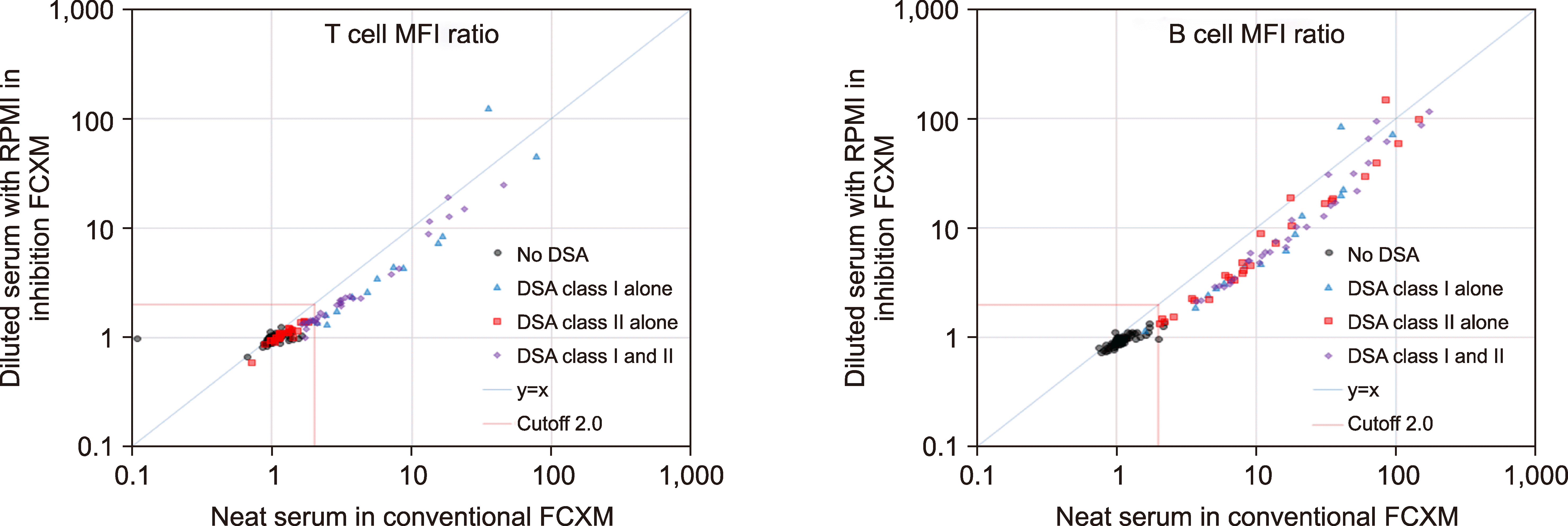 | Fig. 4Dilution effects on inhibition FCXM with RPMI as the diluent. In principle, the MFI ratio of a serum diluted with RPMI should be less than that of the neat serum with DSAs, and thus be plotted under the diagonal line, y=x. Most MFI ratios of diluted sera in the inhibition FCXM were lower than those of neat sera in the conventional FCXM. The higher values might be due to the prozone phenomenon (excess antibody).
Abbreviations: DSA, donor-specific HLA alloantibody; FCXM, flow cytometric crossmatch; MFI, mean fluorescence intensity.
|
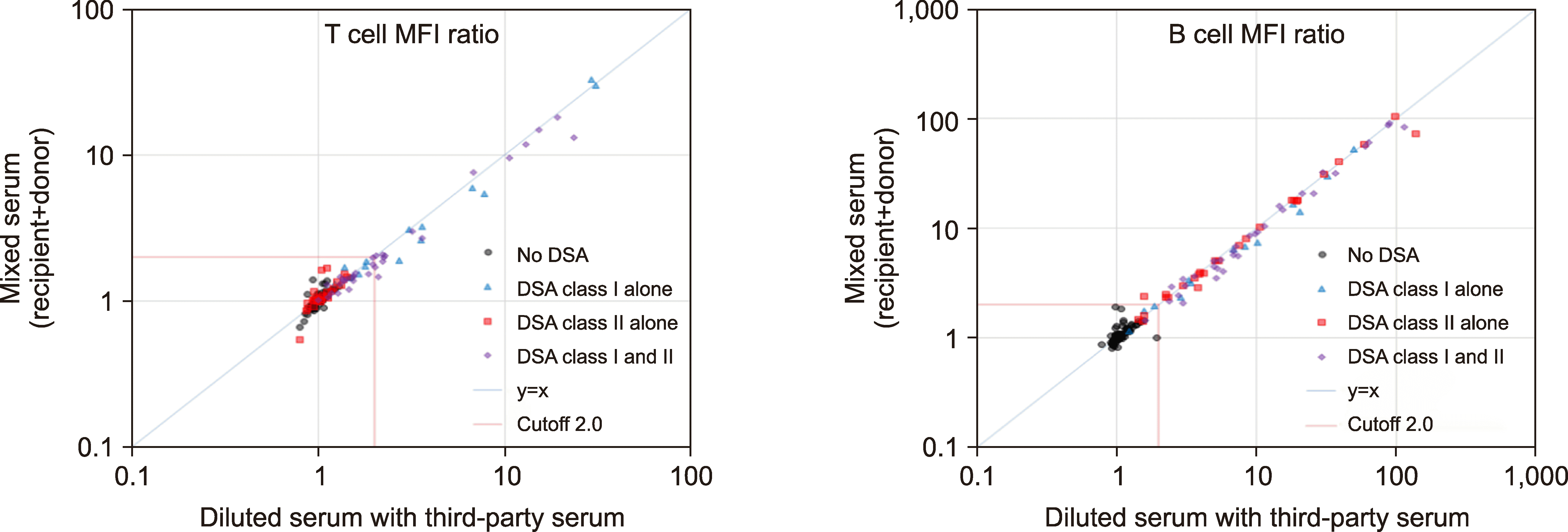 | Fig. 5The neutralizing effects of donor sera compared to third-party sera as diluents in inhibition FCXM. When the neutralizing effects of the sera of previous sensitizers are evident, their MFI ratios should be less than those generated by the sera diluted with third-party sera and thus should be plotted under the diagonal line, y=x. In the group with both class I and class II DSAs (N=33), neutralizing effects were evident in both T cell and B cell FCXM. In the group with any positive DSAs (class I or class II DSAs, N=74), neutralizing effects were evident in the B cell FCXM only.
Abbreviations: DSA, donor-specific HLA alloantibody; FCXM, flow cytometric crossmatch; MFI, mean fluorescence intensity.
|
Table 3
| Donor | N |
T cell MFI ratio, mean±SD, median (range) |
B cell MFI ratio, mean±SD, median (range) |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Inhibition FCXM |
Conventional FCXM (neat recipient serum) |
Inhibition FCXM |
Conventional FCXM (neat recipient serum) |
||||||
|
|
|
||||||||
| Recipient serum diluted with |
Mixed serum (recipient+donor) |
Recipient serum diluted with |
Mixed serum (recipient+donor) |
||||||
|
|
|
||||||||
| RPMI | Third-party serum | RPMI | Third-party serum | ||||||
| Previous sensitizer | |||||||||
| Negative DSA | 69 | 0.97±0.09 | 1.01±0.11 | 1.03±0.15 | 1.05±0.20 | 0.96±0.13 | 1.09±0.18 | 1.10±0.21 | 1.14±0.30 |
|
0.98▼a) (0.66–1.25) |
1.00 (0.79–1.50) |
1.03 (0.67–1.44) |
1.00 (0.11–1.63) |
0.95▼ (0.73–1.37) |
1.04 (0.79–1.95) |
1.06 (0.81–1.93) |
1.05 (0.75–2.20) |
||
| P<0.00005 | P=0.0894 | P<0.0000001 | P=0.5757 | ||||||
| DSA class I alone | 14 | 15.13±33.76 | 6.93b)±10.01 | 6.74±10.50 | 13.38±20.58 | 17.84±26.92 | 11.74±14.01 | 10.87±14.26 | 22.38±25.20 |
|
3.04▲ (1.33–125.39) |
2.88 (1.23–30.81) |
2.25 (1.31–32.51) |
5.28 (1.95–77.68) |
5.48▼ (1.16–85.62) |
6.00 (1.23–49.33) |
5.76 (1.16–52.46) |
13.54 (1.61–94.26) |
||
| P<0.01 | P=0.1671 | P<0.05 | P=0.0962 | ||||||
| DSA class II alone | 27 | 1.07±0.18 | 1.07±0.17 | 1.14±0.25 | 1.24±0.27 | 18.99±33.19 | 18.94±32.01 | 16.56±24.90 | 26.10±36.15 |
|
1.07 (0.59–1.39) |
1.04▼ (0.79–1.42) |
1.06 (0.54–1.67) |
1.16 (0.71–1.83) |
4.50 (1.34–145.98) |
5.08 (1.44–138.47) |
5.03 (1.41–103.53) |
8.14 (2.06–144.92) |
||
| P=0.0652 | P<0.05 | P=0.5561 | P=0.2276 | ||||||
|
Both DSA classes (class I and II) |
33 | 4.32±5.78 | 4.13±5.61 | 3.60±4.53 | 6.38±9.06 | 21.40±29.51 | 20.57±28.98 | 18.88±26.14 | 32.39±40.12 |
|
1.97▲ (0.99–24.77) |
1.96▲ (1.00–23.41) |
1.55 (1.01–18.10) |
3.09 (1.65–45.78) |
7.49▲ (2.15–114.56) |
7.16▲ (1.60–114.28) |
6.19 (1.46–91.02) |
16.19 (3.74–173.15) |
||
| P<0.00001 | P<0.0005 | P<0.0005 | P<0.005 | ||||||
|
Any DSA (class I or II) |
74 | 5.18±15.60 | 3.54±6.02 | 3.30±5.72 | 5.83±11.42 | 19.85±30.09 | 18.30±27.91 | 16.52±23.78 | 28.20±36.06 |
|
1.40▼ (0.59–125.39) |
1.40▲ (0.79–30.81) |
1.46 (0.54–32.51) |
1.99 (0.71–77.68) |
6.17▲ (1.16–145.98) |
6.80▲ (1.23–138.47) |
5.60 (1.16–103.53) |
12.09 (1.61–173.15) |
||
| P<0.0001 | P<0.05 | P<0.0005 | P<0.0005 | ||||||
| Total | 143 | 3.15±11.38 | 2.32±4.50 | 2.20±4.26 | 3.52±8.53 | 10.73±23.56 | 10.00±21.79 | 9.08±18.72 | 15.24±29.55 |
|
1.04 (0.59–125.39) |
1.06 (0.79–30.81) |
1.11 (0.54–32.51) |
1.18 (0.11–77.68) |
1.40 (0.73–145.98) |
1.56▼ (0.79–138.47) |
1.60 (0.81–103.53) |
2.11 (0.10–173.15) |
||
| P=0.4435 | P=0.2897 | P=0.5126 | P<0.001 | ||||||
| Non-sensitizer | 6 | 1.00±0.13 | 1.05±0.08 | 1.03±0.13 | 1.14±0.25 | 1.62±0.78 | 1.79±0.75 | 1.71±0.78 | 1.14±0.19 |
|
0.99 (0.84–1.18) |
1.03 (0.97–1.18) |
1.00 (0.90–1.18) |
1.01 (0.95–1.59) |
1.68 (0.64–2.91) |
1.72 (1.04–3.17) |
1.65 (0.92–3.12) |
1.13 (0.83–1.42) |
||
| P=0.1441 | P=0.2785 | P=0.2476 | P=0.1411 | ||||||
a)The symbols ▼and ▲indicate a significantly decreased or increased median value, respectively, when the MFI ratios of diluted sera were compared to those of mixed sera. b)Bold fonts indicate the most efficient comparisons between the diluted serum and the mixed serum to reveal neutralization effects with minimal matrix effects.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download