Abstract
Background
Immune checkpoint inhibitors have demonstrated efficacy in the treatment of classical Hodgkin’s lymphoma (cHL). We analyzed the efficacy and safety of pembrolizumab or nivolumab in patients with pretreated cHL.
Methods
Clinical data from the cancer chemotherapy registry of Samsung Medical Center were retrospectively analyzed to study patients with cHL treated with pembrolizumab or nivolumab between Oct 2015 and Dec 2018.
Results
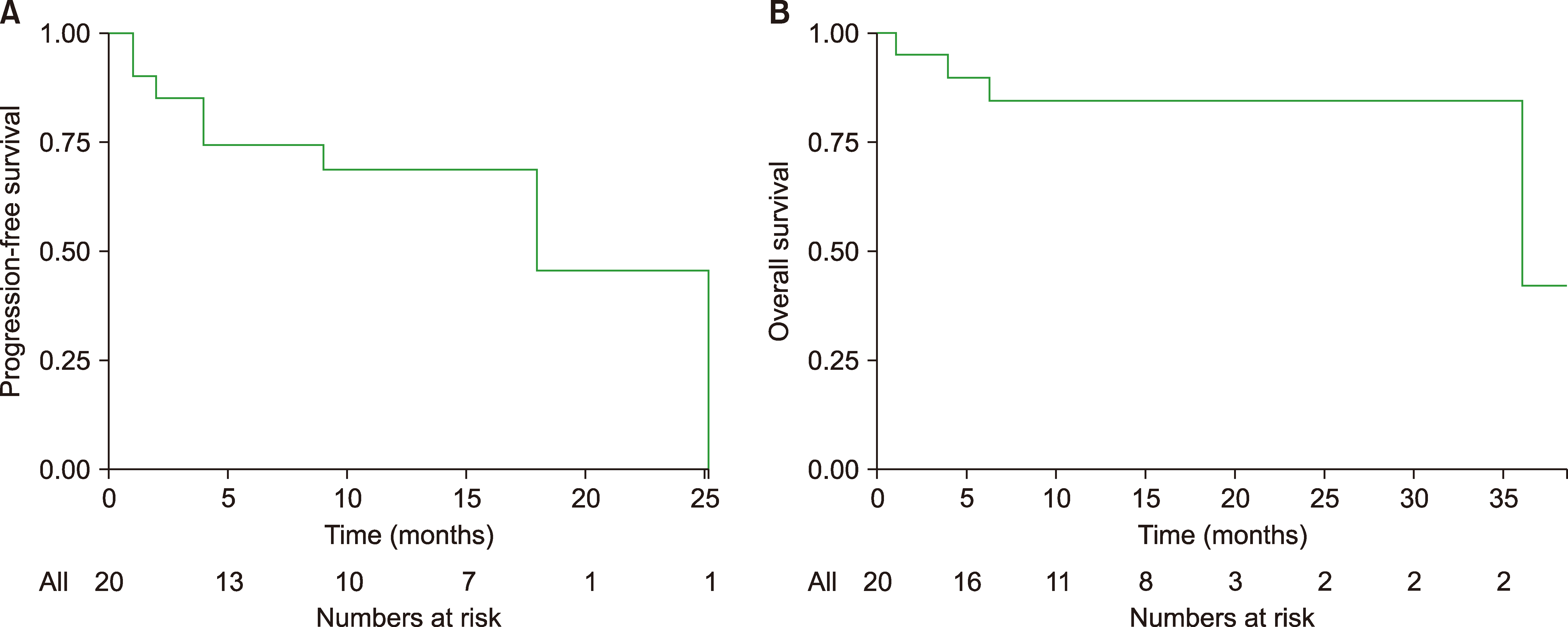
Of the 20 patients, seven (35%) were enrolled in the study after a relapse following autologous hematopoietic stem cell transplantation (ASCT) and 12 (60%) after a relapse following receipt of brentuximab vedotin (BV). Sixteen (80%) patients received pembrolizumab, and four (20%) patients received nivolumab. The complete remission rate was 45% (9/20), and 30% (6/20) of patients achieved partial remission, for an overall response rate (RR) of 75% [15/20; 95% confidence interval (CI), 34.7‒93.3]. With a median follow-up duration of 14 months, the median PFS was 18 months (95% CI, 2.4‒33.5 mo), and the median OS was 36 months [95% CI, 36-not applicable (NA) mo]. Pembrolizumab and nivolumab were generally well tolerated.
Go to : 
The incidence of classical Hodgkin’s lymphoma (cHL) has been reported to be 0.4 per 100,000 person-years, constituting approximately 6% of all lymphomas in Korea [1]. Although the incidence of cHL was lower in Korea than in Western countries, the distributions of histological subtypes were similar [1]. To prevent T-cells from damaging normal cells, several immune system checkpoints exist, where undesirable immune responses can be inhibited or blocked [2]. One inhibitory immune checkpoint is the programmed cell death 1 (PD-1) pathway. Recently, PD-1 inhibitors including nivolumab and pembrolizumab were evaluated for treatment of patients with cHL showing disease progression during or following first-line chemotherapy [3]. The impressive results of nivolumab in relapsed and refractory cHL led to the U.S. Food and Drug Administration (FDA) approval in 2016 [4, 5]. Prior to nivolumab, brentuximab vedotin (BV) was the only FDA approved cHL therapy after failure of autologous hematopoietic stem cell transplantation (ASCT), and there were no approved cHL therapies after failure of both ASCT and BV [6]. Another anti-PD-1 monoclonal antibody, pembrolizumab, was studied in a phase 1 trial published in 2016 (Keynote 013) [7]. A single-arm, phase II study of pembrolizumab in patients with relapsed or refractory cHL (KEYNOTE-087) showed that pembrolizumab was associated with high response rates and an acceptable safety profile in patients with relapsed or refractory cHL [8].
We analyzed the efficacy and safety of pembrolizumab or nivolumab in patients with cHL after previous chemotherapy or ASCT at a single cancer center institute. To our knowledge, this is the first real-world study to assess treatment patterns and outcomes in Korean patients with cHL.
Go to : 
This study using the cancer registry of Samsung Medical Center (SMC) was retrospectively reviewed and approved by the SMC Institutional Review Board (Seoul, Korea). All patients gave written informed consent prior to starting therapy, according to institutional guidelines. We reviewed the medical records of patients with cHL who were treated with pembrolizumab or nivolumab as second-line or later therapy between Oct 2015 and Dec 2018. The eastern cooperative oncology group (ECOG) performance status was also assessed. Criteria for study inclusion were as follows: 1) histologically confirmed diagnosis of cHL arising from lymph node and/or extranodal organs, 2) 18 years of age or older, and 3) ASCT or prior chemotherapy such as ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) and BV.
Pembrolizumab 100 or 200 mg was administered intravenously every three weeks, and nivolumab 3 mg/kg was administered intravenously every two weeks. During the treatment period, laboratory and imaging assessments were performed. The laboratory assessments included complete blood count (CBC) with differential, serum urea, serum creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, alkaline phosphatase, lactate dehydrogenase (LDH), and Epstein-Barr virus (EBV). The immunohistochemistry (IHC) staining of programmed cell death - ligand 1 (PD-L1) 22C3 using monoclonal mouse anti-PD-L1 clone 22C3 was reviewed. The PD-L1 tumor proportion score (TPS) was calculated as the percentage of viable tumor cells showing partial or complete membrane staining at any intensity. Positron emission tomography (PET) and computed tomography (CT) scans of all involved sites were evaluated before starting PD-1 inhibitors. To evaluate clinical response to nivolumab or pembrolizumab, patients underwent CT or PET scans at weeks 12, 24, and 36, or 60 weeks. We evaluated efficacy assessments using the revised response criteria for malignant lymphomas [9]. The best overall response was defined as the best response between the date of the first dose and the last efficacy assessment before subsequent therapy. Pembrolizumab or nivolumab was continued until complete response, disease progression, or excessive toxic effects.
The primary endpoint of the present study was overall response rate (RR). Secondary endpoints were progression-free survival (PFS), overall survival (OS), and safety. The time from the first day of pembrolizumab or nivolumab administration to the date of documented disease progression or death was considered PFS. The time from the first day of pembrolizumab or nivolumab administration to the date of death was considered OS. Cox proportional hazards model was used in univariate analysis to identify significant prognostic factors for PFS and OS. All P-values were two-sided, with P<0.05 indicating statistical significance. All analyses were performed using R for Windows v3.3.2 software (R Core Team, Vienna, Austria; http://www.r-project.org).
Go to : 
Patient characteristics are given in Table 1. As shown, among the 20 patients, 65% were men, and the median age was 34 years (range, 22–79). There were 4 patients aged 65 or older. Medical records from 16 eligible patients who were treated with pembrolizumab and 4 patients with nivolumab for relapsed or refractory cHL were reviewed. Of those treated with pembrolizumab, 10 had been treated with pembrolizumab 100 mg every 3 weeks and 6 with pembrolizumab 200 mg. Efficacy did not differ significantly between the doses. Of 20 patients, most had been treated with ABVD chemotherapy (90%), 12 (60%) received BV before immunotherapy, and 7 (35%) had relapsed/refractory disease after ASCT. Only 3 patients had available data on PD-L1 expression: one patient with PD-L1 (22C3) 100%, and the others with PD-L1 (22C3) 50% and 40%, respectively. Extranodal disease involving lung, bone, liver, or pleura was found in 70% of the patients (14/20).
Patient characteristics.
Patients received pembrolizumab for a total of 171 cycles (median, 13; range, 1–24) and nivolumab for 67 cycles (median, 15; range, 5–24). Of 20 patients, nine showed complete responses to immunotherapy (45%) and six showed partial responses (30%). The objective response rate was 75% [15/20; 95% confidence interval (CI), 34.7–93.3]. The maximum reduction in tumor burden from baseline for each patient is shown in Fig. 1A. Of 9 patients with complete response, 7 had received previous treatment with brentuximab. Of the 15 patients who had a complete or partial response, 4 (26.6%) had the first response by 10 weeks (range, 8 to 16 wk) (Fig. 1B). At the time of data collection with a median follow-up duration of 14 months, the median PFS was 18 months (95% CI, 2.4–33.5, Fig. 2A), and the median OS was 36 months (95% CI, 36-NA mo, Fig. 2B).
Of the 11 patients (55%) who discontinued treatment, 2 (10%) had complete remission, 1 (5%) had toxic effects (cardiac myocarditis), 7 (35%) had progressive disease during treatment, and 1 (5%) elected to undergo ASCT. Two patients showed complete remission after pseudoprogression when treated with pembrolizumab. Univariate analysis results for PFS and OS are shown in Table 2. PFS was not significantly influenced by age, performance status, EBV, prior ASCT, or type of PD-1 inhibitors (pembrolizumab vs. nivolumab). Univariate analysis revealed that ECOG performance status (0–1 vs. 2) was a significant prognostic factor for overall survival (P=0.016).
Univariate analysis of prognostic factors for survival.
Pembrolizumab or nivolumab was generally well tolerated. The most common treatment-related adverse events (AEs) (Table 3) were fever (15%), aminotransferase increase (10%), fatigue (5%), and neutropenia (5%). Immune-related AEs resulting in treatment discontinuation were observed in 2 patients who both received steroids. Of 20 patients, one had pembrolizumab-associated myocarditis. After pembrolizumab was administered for 10 cycles, a 32-year-old woman presented with new, progressive dyspnea that had developed during the previous week. Echocardiography revealed pericardial and pleural effusion. Heart biopsy revealed lymphohistiocytic infiltration with myocyte damage, consistent with lymphocytic myocarditis. She recovered rapidly after steroid therapy. There were no grade 4 treatment-related AEs and no deaths related to immunotherapy.
Go to : 
This study analyzed the clinical outcomes of PD-1 immunotherapy in patients with cHL who had been treated with previous chemotherapy or ASCT. Although conventional therapy (i.e., ABVD, BV) for cHL is associated with a high rate of cure (80%), up to 20% of patients will have relapsed or refractory disease [10]. In heavily pretreated patients with relapsed or refractory cHL, the majority of whom experienced relapse after ASCT and BV treatment, the use of nivolumab was associated with an overall response rate of 66–87% and a rate of PFS of 77–86% at 24 weeks [5, 11]. We await the results of randomized studies such as the ongoing phase III study comparing brentuximab with nivolumab in combination versus brentuximab alone in patients with relapsed/refractory cHL (NCT03138499). Several ongoing trials are investigating the efficacy of pembrolizumab in various settings, including a phase III head-to-head trial of pembrolizumab and brentuximab in patients with relapsed/refractory cHL (NCT 02684292).
As far as we know, this is the first real world study of PD-1 inhibitors in Korean patients with cHL. In 2015, when immunotherapy was introduced into clinical practice of cHL, some patients with cHL in this study were treated with low-dose pembrolizumab. A previous study showed that low-dose pembrolizumab (median dose 100 mg; range, 100–200 mg, every 3 wk) was highly efficacious, achieving responses with minimal toxicity and at lower costs [12].
Some patients who has an increase in the size of lesions at response assessment were noted to have late response [13]. The previous studies described these unique response patterns, termed pseudoprogression [14]. An analysis of pembrolizumab in patients with advanced melanoma found that 3.6% (seven of 192 patients) experienced progressive disease at first assessment, followed by clinical response at second assessment [15]. In this study, two patients had definite clinical improvement despite imaging data indicating progression. Clinicians should be aware of the potential for pseudoprogression in patients showing clinical improvement.
Seven (35%) patients had progressive disease during treatment. One of them was transformed into diffuse large B cell lymphoma (DLBCL) and treated with RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). Of seven patients, two received ASCT after PD-1 inhibitors, but both showed progression. Three patients were treated with another PD-1 inhibitor, and one patient died of aspiration pneumonia.
The exact mechanism of PD-1 inhibitors in cHL remains unknown, and more research is needed. In cHL, alterations in chromosome 9p24.1 increase the abundance of PD-1 ligands and promote their induction through the Janus kinase–signal transducer and activator of transcription signaling, defining the PD-1 pathway as a rational therapeutic target [16].
Immune checkpoint blockade can have adverse inflammatory effects, which are often termed immune-related adverse events, involving the gastrointestinal tract, endocrine glands, skin, liver, lung, and heart [17]. Immune-mediated pneumonitis, colitis, hepatitis, hypophysitis, and thyroiditis are less common toxic effects of PD-1 antibody [18]. Surprisingly, there was a life-threatening or fatal treatment-related event (myocarditis) in this study. She is now regularly watching the progress without further chemotherapy with a complete remission. There are rare cases of immune checkpoint inhibitor-induced myocarditis [19]. As a result of recent data, in heavily pretreated disease, immune checkpoint inhibitors are moving to earlier lines of treatment [20].
In this study, pembrolizumab or nivolumab demonstrated clinical efficacy and tolerability in patients with cHL who failed previous chemotherapy or ASCT. Further investigations of immune checkpoint inhibitors and combinatory approaches in relapsed or refractory cHL are needed.
Go to : 
ACKNOWLEDGMENTS
This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Seok Jin Kim and Won Seog Kim contributed to the study conception and design. Joon Young Hur and Sang Eun Yoon contributed to revision of the manuscript. Joon Young Hur contributed to acquisition of data and drafting of manuscript. All authors read and approved the final manuscript.
Go to : 
Notes
Authors’ Disclosures of Potential Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Go to : 
REFERENCES
1. Won YW, Kwon JH, Lee SI, et al. 2012; Clinical features and outcomes of Hodgkin's lymphoma in Korea: Consortium for Improving Survival of Lymphoma (CISL). Ann Hematol. 91:223–33. DOI: 10.1007/s00277-011-1297-x. PMID: 21789622.

2. De Re V, Caggiari L, Repetto O, Mussolin L, Mascarin M. 2019; Classical Hodgkin's lymphoma in the era of immune checkpoint inhibition. J Clin Med. 8:1596. DOI: 10.3390/jcm8101596. PMID: 31581738. PMCID: PMC6832444.

3. Bröckelmann PJ, Engert A. 2017; Checkpoint inhibition in Hodgkin lymphoma - a review. Oncol Res Treat. 40:654–60. DOI: 10.1159/000481800. PMID: 29065424.

4. Kasamon YL, de Claro RA, Wang Y, Shen YL, Farrell AT, Pazdur R. 2017; FDA approval summary: nivolumab for the treatment of relapsed or progressive classical Hodgkin lymphoma. Oncologist. 22:585–91. DOI: 10.1634/theoncologist.2017-0004. PMID: 28438889. PMCID: PMC5423515.

5. Ansell SM, Lesokhin AM, Borrello I, et al. 2014; PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 372:311–9. DOI: 10.1056/NEJMoa1411087. PMID: 25482239. PMCID: PMC4348009.

6. de Claro RA, McGinn K, Kwitkowski V, et al. 2012; U.S. Food and Drug Administration approval summary: brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res. 18:5845–9. DOI: 10.1158/1078-0432.CCR-12-1803. PMID: 22962441.

7. Armand P, Shipp MA, Ribrag V, et al. 2016; Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma After brentuximab vedotin failure. J Clin Oncol. 34:3733–9. DOI: 10.1200/JCO.2016.67.3467. PMID: 27354476. PMCID: PMC5791838.

8. Chen R, Zinzani PL, Fanale MA, et al. 2017; Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 35:2125–32. DOI: 10.1200/JCO.2016.72.1316. PMID: 28441111. PMCID: PMC5791843.

9. Cheson BD, Pfistner B, Juweid ME, et al. 2007; Revised response criteria for malignant lymphoma. J Clin Oncol. 25:579–86. DOI: 10.1200/JCO.2006.09.2403. PMID: 17242396.
10. Bair SM, Mato A, Svoboda J. 2018; Immunotherapy for the treatment of Hodgkin lymphoma: an evolving paradigm. Clin Lymphoma Myeloma Leuk. 18:380–91. DOI: 10.1016/j.clml.2018.03.012. PMID: 29685424.

11. Younes A, Santoro A, Shipp M, et al. 2016; Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 17:1283–94. DOI: 10.1016/S1470-2045(16)30167-X. PMID: 27451390. PMCID: PMC5541855.

12. Chan TSY, Luk TH, Lau JSM, Khong PL, Kwong YL. 2017; Low-dose pembrolizumab for relapsed/refractory Hodgkin lymphoma: high efficacy with minimal toxicity. Ann Hematol. 96:647–51. DOI: 10.1007/s00277-017-2931-z. PMID: 28138786.

13. Seymour L, Bogaerts J, Perrone A, et al. 2017; iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 18:e143–52. DOI: 10.1016/S1470-2045(17)30074-8. PMID: 28271869. PMCID: PMC5648544.

14. Hong J, Bae J, Lee SG, et al. 2017; Complete Remission after pseudoprogression in refractory classical Hodgkin lymphoma treated with pembrolizumab. Korean J Med. 92:415–8. DOI: 10.3904/kjm.2017.92.4.415.

15. Chiou VL, Burotto M. 2015; Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 33:3541–3. DOI: 10.1200/JCO.2015.61.6870. PMID: 26261262. PMCID: PMC4622096.

16. Green MR, Monti S, Rodig SJ, et al. 2010; Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 116:3268–77. DOI: 10.1182/blood-2010-05-282780. PMID: 20628145. PMCID: PMC2995356.

17. Postow MA, Sidlow R, Hellmann MD. 2018; Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 378:158–68. DOI: 10.1056/NEJMra1703481. PMID: 29320654.

18. Topalian SL, Hodi FS, Brahmer JR, et al. 2012; Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 366:2443–54. DOI: 10.1056/NEJMoa1200690. PMID: 22658127. PMCID: PMC3544539.
19. Mahmood SS, Fradley MG, Cohen JV, et al. 2018; Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 71:1755–64. DOI: 10.1016/j.jacc.2018.02.037. PMID: 29567210. PMCID: PMC6196725.

20. Vassilakopoulos TP, Chatzidimitriou C, Asimakopoulos JV, et al. 2019; Immunotherapy in Hodgkin lymphoma: present status and future strategies. Cancers (Basel). 11:1071. DOI: 10.3390/cancers11081071. PMID: 31362369. PMCID: PMC6721364.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download