INTRODUCTION
MATERIALS AND METHODS
Patients
Protocol of FM-MP therapy
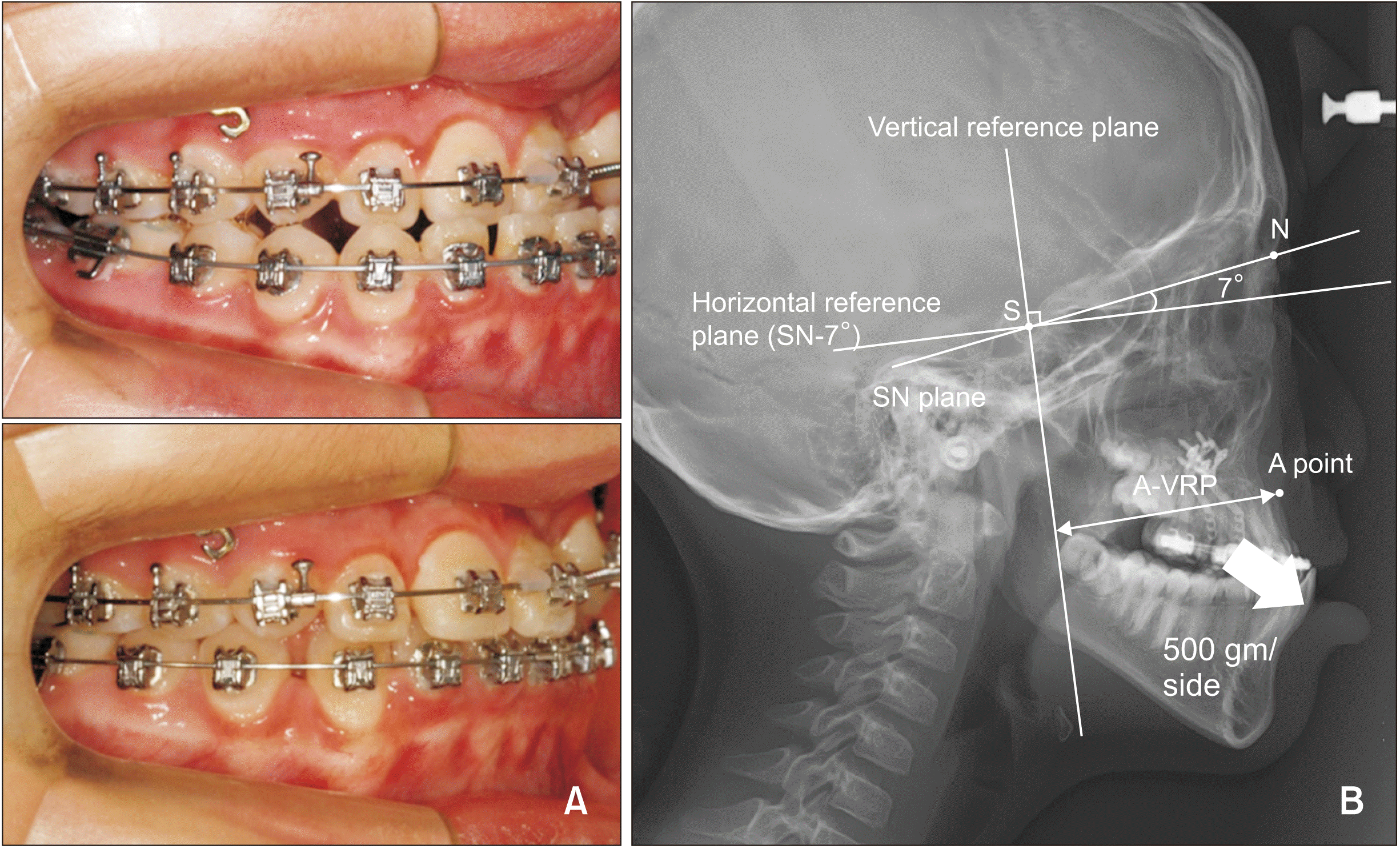 | Figure 1
A, An example of facemask with miniplate (FM-MP) therapy. B, Reference lines. The horizontal reference plane (HRP) passes the Sella (S) at an angle of 7 degree clockwise to the Sella-Nasion (SN) plane. The vertical reference plane (VRP) is perpendicular to the HRP passing through the S. A-VRP is the horizontal distance from the VRP to A point. |
Cephalometric analysis
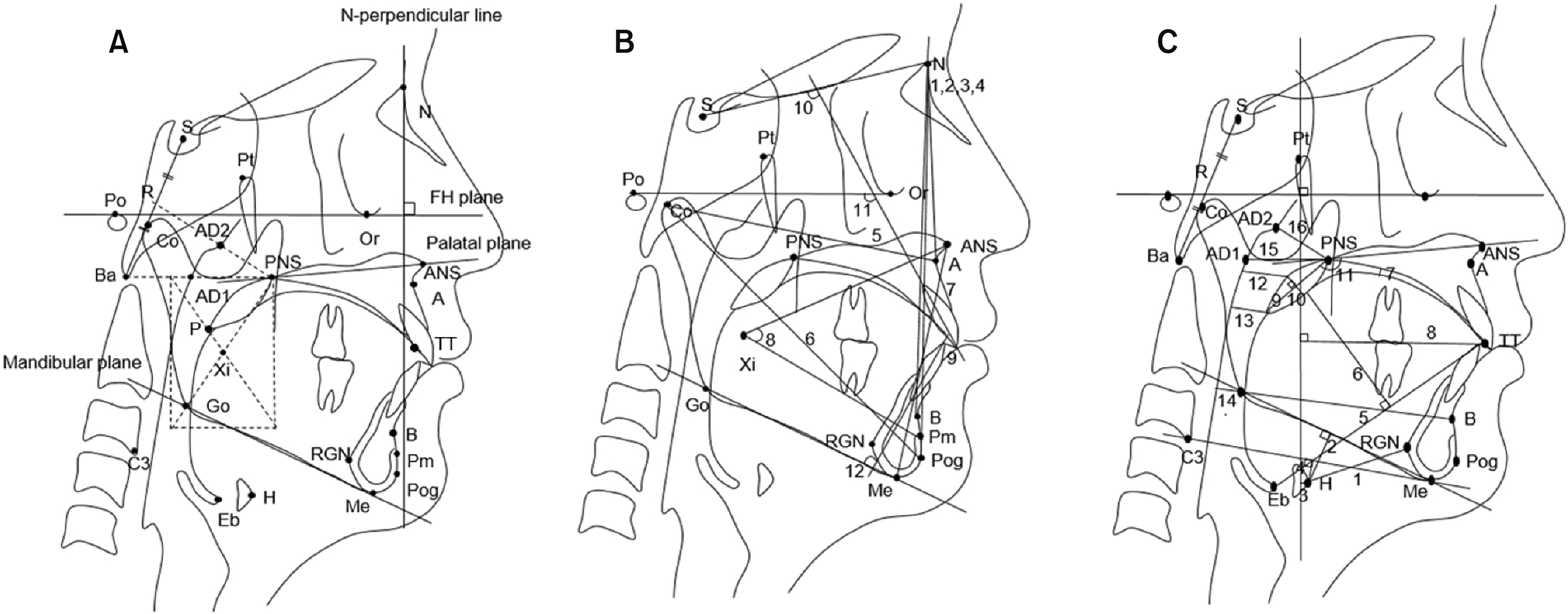 | Figure 2Cephalometric landmarks and measurements. A, Landmarks. S, Sella; N, nasion; Ba, basion; Or, orbitale; Po, porion; Pt, pterygoid point; R, midpoint between sella and basion; AD1, adenoid point 1 (adenoid tissue on the PNS-Ba line); AD2, adenoid point 2 (adenoid tissue on the R-PNS line); Co, condylion; Go, gonion; ANS, anterior nasal spine; PNS, posterior nasal spine; A, point A; B, point B; TT, tongue tip; Pog, pogonion; Me, menton; P, tip of the soft palate; Eb, base of the epiglottic fold; H, hyoidale (the most anterosuperior point on the body of the hyoid bone); RGN, retrognathion; Pm, protuberance menti; Xi, geometric center of the ramus; FH, Frankfort horizontal; C3, the third vertebrae. B, Skeletodental variables. 1, SNA; 2, SNB; 3, SN-Pog; 4, ANB; 5, effective maxillary length (EMxL); 6, effective mandibular length (EMnL); 7, ANS-Me; 8, lower facial height (ANS-Xi-Pm); 9, overjet; 10, U1-SN; 11, U1-FH; 12, L1-MP. C, Airway-related variables. hyoid bone 1, H-RGN; 2, MP-H; 3, H-PTV; 4, H-C3Me; tongue 5, TGL; 6, TGH; 7, Td-PP; 8, TT-PTV; soft palate 9, SPL; 10, SPT; 11, SPA; pharyngeal airway 12, SPAS; 13, MAS; 14, IAS; 15, PNS-AD1; 16, PNS-AD2.
See Table 2 for definitions of each landmark or measurement.
|
Table 2
Statistical analysis
RESULTS
Comparison of the demographic data and cephalometric variables at T0
Table 3
See Table 2 for definitions of each landmark or measurement.
Evaluation of the change in cephalometric variables during T0–T1 in each group (Table 4)
Table 4
| Variable | Group 1 | Group 2 | p-value‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Mean | SD | p-value† | Mean | SD | p-value† | ||||
| Skeletodental | ΔA-VRP (mm) | 6.07 | 1.77 | 0.002** | 1.29 | 0.85 | 0.001** | 0.000*** | |
| ΔSNA (°) | 2.53 | 1.22 | 0.000*** | 0.54 | 0.75 | 0.330 | 0.000*** | ||
| ΔA-N perp (mm) | 2.70 | 1.14 | 0.000*** | 1.04 | 0.92 | 0.874 | 0.000*** | ||
| ΔPNS-HRP (mm) | 4.52 | 1.79 | 0.000*** | 2.86 | 1.72 | 0.000*** | 0.030* | ||
| ΔPNS-VRP (mm) | 4.76 | 0.82 | 0.000*** | 1.14 | 0.80 | 0.070 | 0.000*** | ||
| ΔSNB (°) | 0.18 | 2.13 | 0.771 | 0.64 | 2.35 | 0.365 | 0.377 | ||
| ΔB-N perp (mm) | 1.31 | 2.23 | 0.067 | 1.62 | 2.79 | 0.070 | 0.767 | ||
| ΔSN-Pog (°) | 0.37 | 2.34 | 0.598 | 0.13 | 2.39 | 0.860 | 0.805 | ||
| ΔPog-N perp (mm) | 0.31 | 2.35 | 0.660 | 1.42 | 2.91 | 0.119 | 0.313 | ||
| ΔANB (°) | 3.35 | 2.09 | 0.003** | 1.18 | 2.21 | 0.090 | 0.008** | ||
| ΔEMxL (mm) | 5.97 | 2.81 | 0.000*** | 2.59 | 1.59 | 0.002** | 0.014* | ||
| ΔEMnL (mm) | 8.80 | 5.62 | 0.000*** | 8.32 | 5.72 | 0.002** | 0.590 | ||
| ΔPP-FH (°) | −0.70 | 0.77 | 0.109 | 0.22 | 0.79 | 0.364 | 0.143 | ||
| ΔMP-FH (°) | −0.62 | 0.96 | 0.300 | 0.14 | 2.34 | 0.838 | 0.597 | ||
| ΔLFH (°) | 0.23 | 4.07 | 0.851 | 0.63 | 2.79 | 0.448 | 0.777 | ||
| ΔOverjet (mm) | 4.32 | 2.87 | 0.000*** | 2.83 | 1.53 | 0.110 | 0.662 | ||
| ΔU1-SN (°) | 7.54 | 8.53 | 0.011* | 7.44 | 8.16 | 0.009** | 0.977 | ||
| ΔU1-FH (°) | 7.43 | 8.52 | 0.012* | 7.19 | 8.12 | 0.011* | 0.944 | ||
| ΔL1-MP (°) | −1.54 | 6.05 | 0.762 | −2.74 | 4.49 | 0.215 | 0.155 | ||
| Airway space | Hyoid bone | ΔH-RGN (mm) | 2.71 | 4.94 | 0.038* | 0.67 | 4.86 | 0.644 | 0.112 |
| ΔMP-H (mm) | 0.55 | 4.00 | 0.430 | 0.74 | 3.55 | 0.483 | 0.897 | ||
| ΔH-PTV (mm) | 1.01 | 6.29 | 0.347 | 2.02 | 3.13 | 0.047* | 0.052 | ||
| ΔH-C3Me (mm) | 0.26 | 3.03 | 0.769 | 0.96 | 2.76 | 0.252 | 0.560 | ||
| Tongue | ΔTGL (mm) | 12.80 | 5.22 | 0.000*** | 3.54 | 5.15 | 0.137 | 0.000*** | |
| ΔTGH (mm) | 2.44 | 3.02 | 0.317 | 2.09 | 3.82 | 0.085 | 0.803 | ||
| ΔTd-PP (mm) | 0.44 | 2.42 | 0.538 | 0.57 | 3.01 | 0.527 | 0.374 | ||
| ΔTT-PTV (mm) | 5.79 | 3.36 | 0.002** | 2.03 | 2.92 | 0.245 | 0.001** | ||
| Soft palate | ΔSPL (mm) | 1.56 | 3.44 | 0.145 | −0.85 | 2.10 | 0.192 | 0.051 | |
| ΔSPT (mm) | 0.39 | 2.08 | 0.532 | 0.52 | 1.81 | 0.340 | 0.869 | ||
| ΔSPA (°) | −3.81 | 2.76 | 0.002** | −2.23 | 3.32 | 0.340 | 0.219 | ||
| Pharyngeal airway | ΔSPAS (mm) | 4.92 | 3.01 | 0.002** | 2.25 | 1.19 | 0.000*** | 0.002** | |
| ΔMAS (mm) | 3.63 | 2.31 | 0.000*** | 1.86 | 1.76 | 0.004** | 0.046* | ||
| ΔIAS (mm) | 2.00 | 2.72 | 0.027* | 0.29 | 3.58 | 0.786 | 0.200 | ||
| ΔVAL (mm) | 8.87 | 2.83 | 0.000*** | 7.13 | 5.23 | 0.001** | 0.326 | ||
| ΔPNS-ad1 (mm) | 5.96 | 2.29 | 0.000*** | 4.43 | 1.26 | 0.000*** | 0.056 | ||
| ΔPNS-ad2 (mm) | 7.41 | 1.73 | 0.000*** | 4.23 | 1.36 | 0.000*** | 0.000*** | ||
See Table 2 for definitions of each landmark or measurement.
Comparison of the changes in cephalometric variables between groups 1 and 2 (Table 4)
Correlation between skeletodental and upper airway variables (Table 5)
Table 5
| Variable | Pharyngeal airway | Hyoid | Tongue | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| PNS-ad2 (mm) | PNS-ad1 (mm) | SPAS (mm) | MAS (mm) | VAL (mm) | MPH (mm) | H-PTV (mm) | H-C3Me (mm) | TGL (mm) | TT-PTV (mm) | |||
| Maxilla | A point | A-VRP (mm) | 0.667** | 0.297 | 0.569** | 0.476* | 0.159 | −0.047 | −0.101 | −0.042 | 0.544** | 0.573** |
| SNA (°) | 0.518** | 0.282 | 0.591** | 0.550** | 0.194 | −0.103 | −0.253 | −0.070 | 0.545** | 0.488* | ||
| EMxL (mm) | 0.219 | 0.357 | 0.680** | 0.452* | 0.478* | 0.002 | −0.147 | −0.028 | 0.550** | 0.625** | ||
| PNS | PNS-HRP (mm) | 0.461* | 0.408* | 0.551** | 0.470* | −0.559** | −0.028 | −0.070 | −0.068 | 0.579** | 0.568** | |
| PNS-VRP (mm) | 0.654** | 0.342 | 0.510* | 0.397 | 0.118 | −0.142 | −0.046 | −0.176 | 0.546** | 0.652** | ||
| Mandible | SNB (°) | 0.414* | 0.421* | 0.276 | 0.435* | −0.166 | −0.294 | 0.284 | −0.417* | −0.022 | −0.021 | |
| SN-Pog (°) | 0.226 | 0.449* | 0.278 | 0.463* | −0.109 | −0.322 | 0.226 | −0.439* | −0.006 | −0.030 | ||
| EMnL (mm) | 0.015 | 0.247 | 0.309 | 0.363 | 0.441* | 0.073 | −0.091 | −0.115 | 0.396 | 0.446* | ||
| MP-FH (°) | −0.292 | −0.349 | −0.318 | −0.466* | 0.038 | 0.585** | −0.436* | 0.361 | 0.216 | −0.083 | ||
See Table 2 for definitions of each landmark or measurement.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download