INTRODUCTION
MATERIALS AND METHODS
Sample size calculation
Subjects, eligibility criteria, and computed tomography
Table 1
Study design
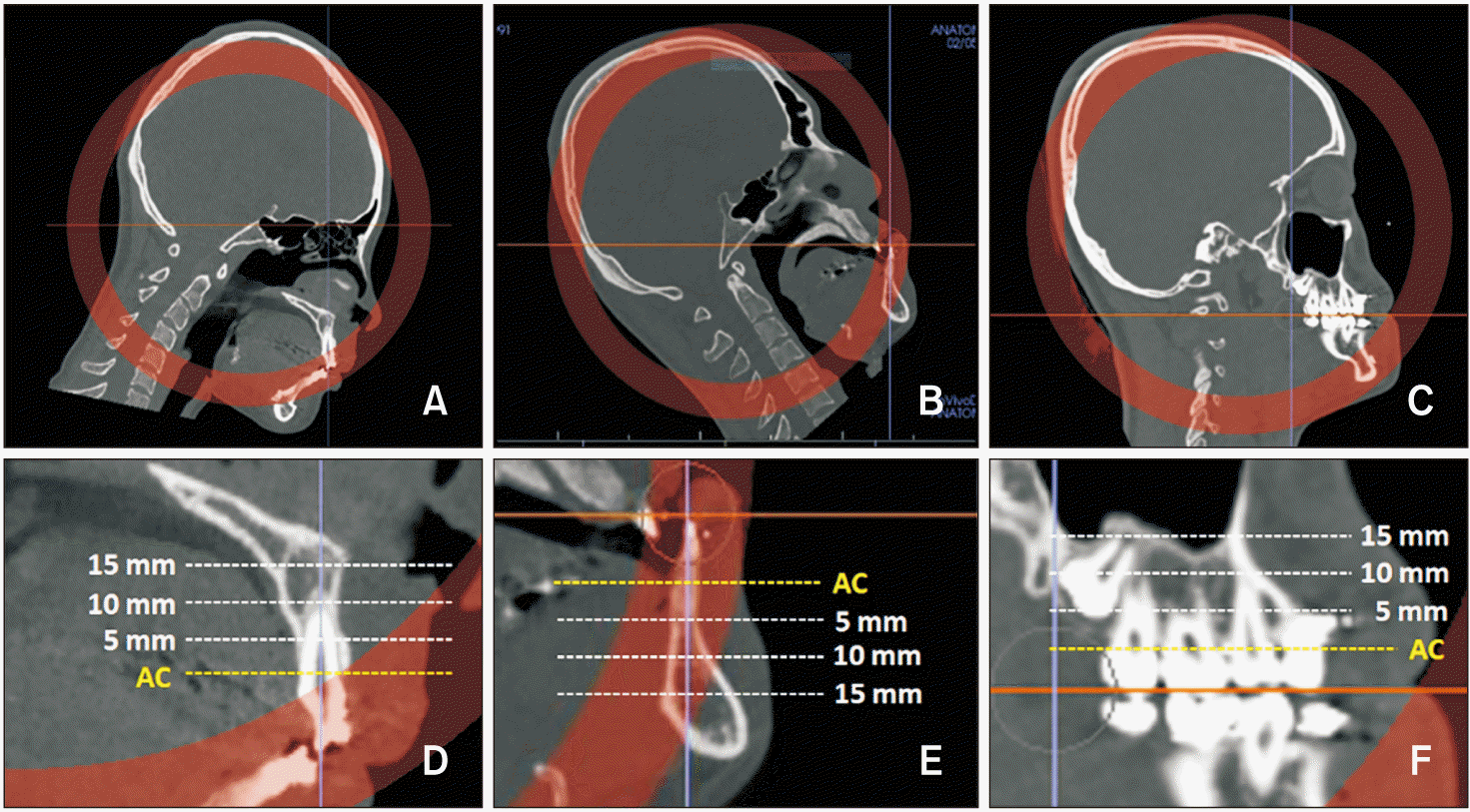 | Figure 2Reorientation and measurement of alveolar bone density in the sagittal views. A, B, Computed tomography images were reoriented to align the axis of the left maxillary and mandibular central incisors perpendicular to the horizontal plane. C, Posterior occlusal plane was aligned so it was parallel to the horizontal plane. D–F, Bone densities were measured at the alveolar crest (AC, yellow line) and at each depth (dotted lines; 5, 10, 15 mm from AC). |
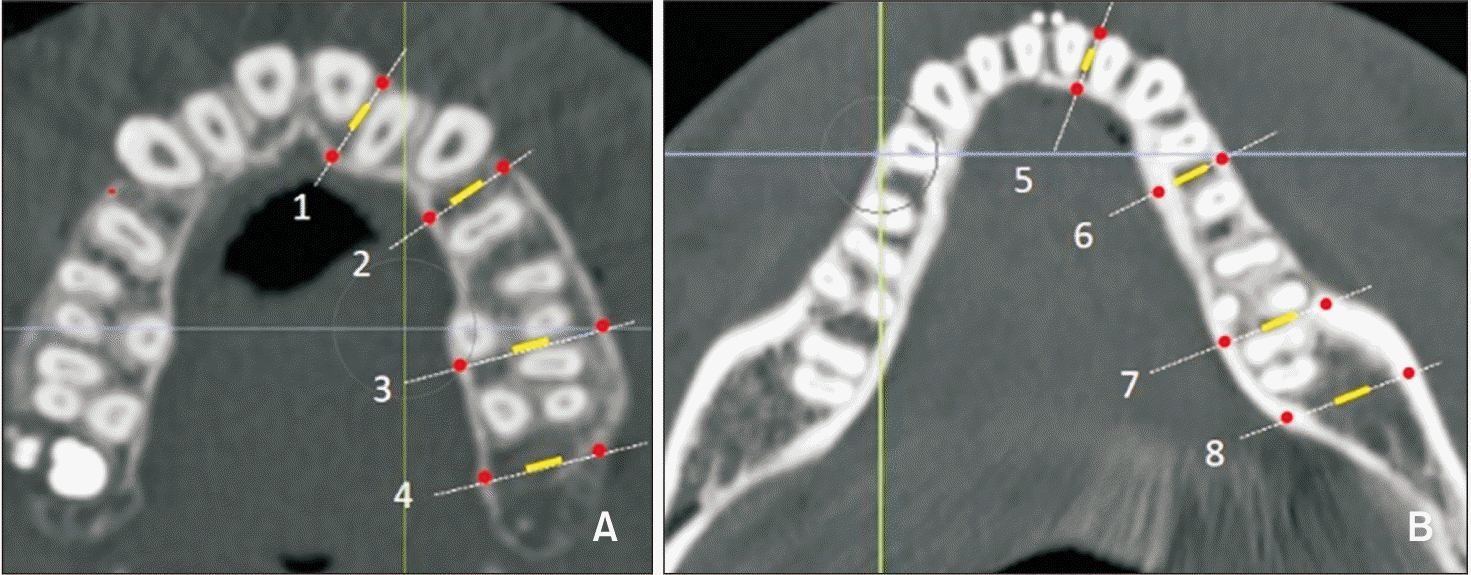 | Figure 3Measurement sites in the alveolar bone. A, B, Measurement sites in the maxilla and mandible. 1, 5: Between central and lateral incisors. 2, 6: Between the first and second premolars or between canines and remaining premolars when premolar extraction was performed. 3, 7: Between the second and first molars. 4: Maxillary tuberosity. 8: Retromolar pad. All measurements were acquired from the left side of the arch. Measurement areas of cortical and cancellous bone are shown as red dots and a yellow line, respectively. |
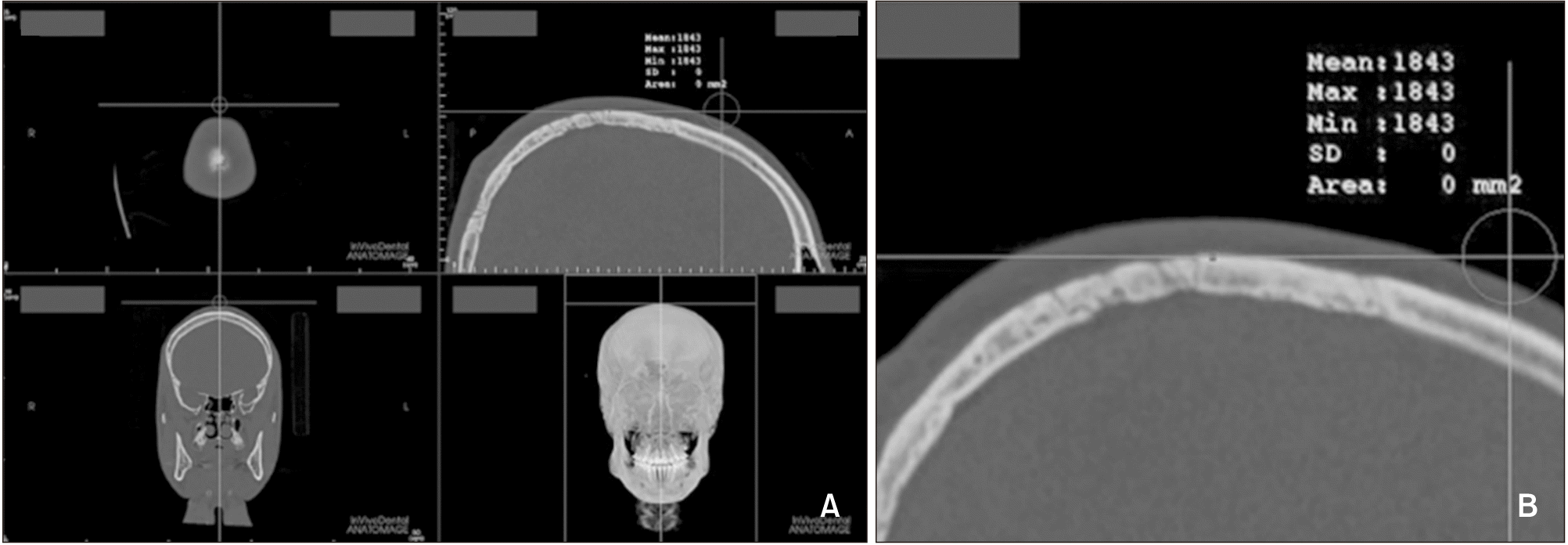 | Figure 4Bone density measurement at the top point of the head. A, The most superior point was selected in the midsagittal view. B, Magnified view of the measurement site. |
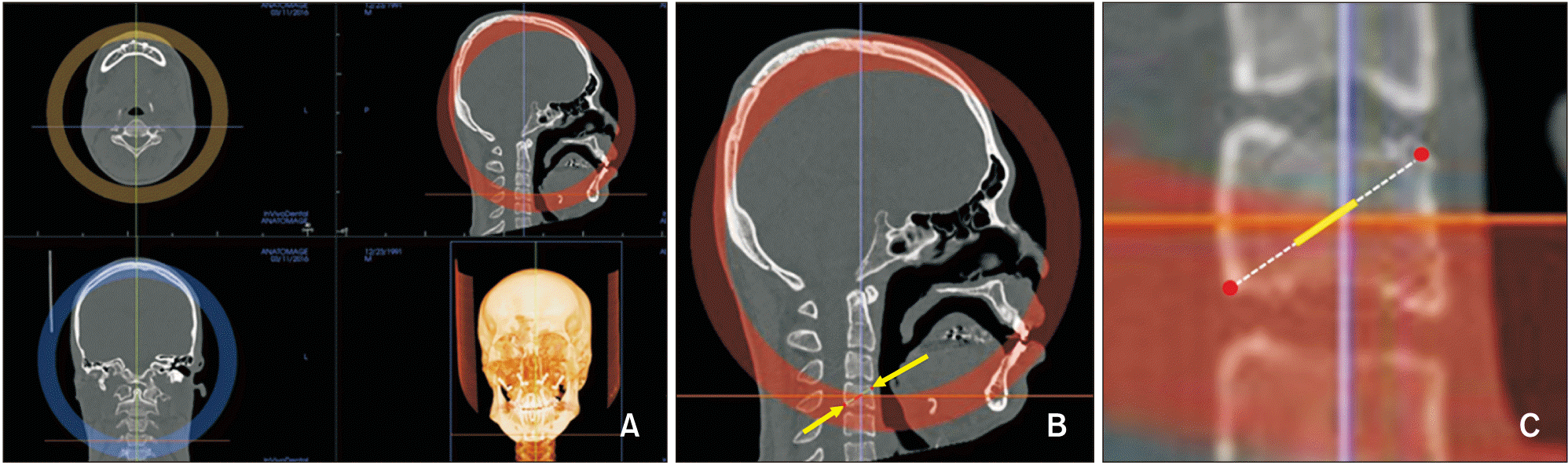
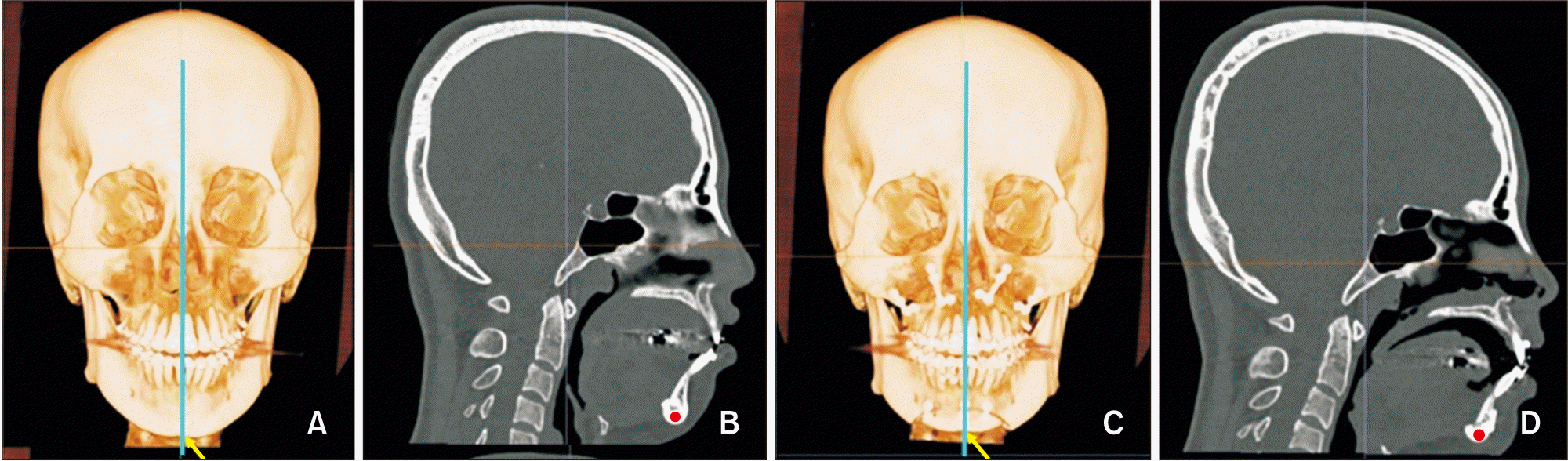 | Figure 5Bone density measurement at menton (Me). A, Presurgical image, the blue line represents the vertical slice drawn over Me (yellow arrow). B, Presurgical sagittal slice at Me (red dot). C, Postsurgical image. D, Postsurgical sagittal slice at Me (red dot). |
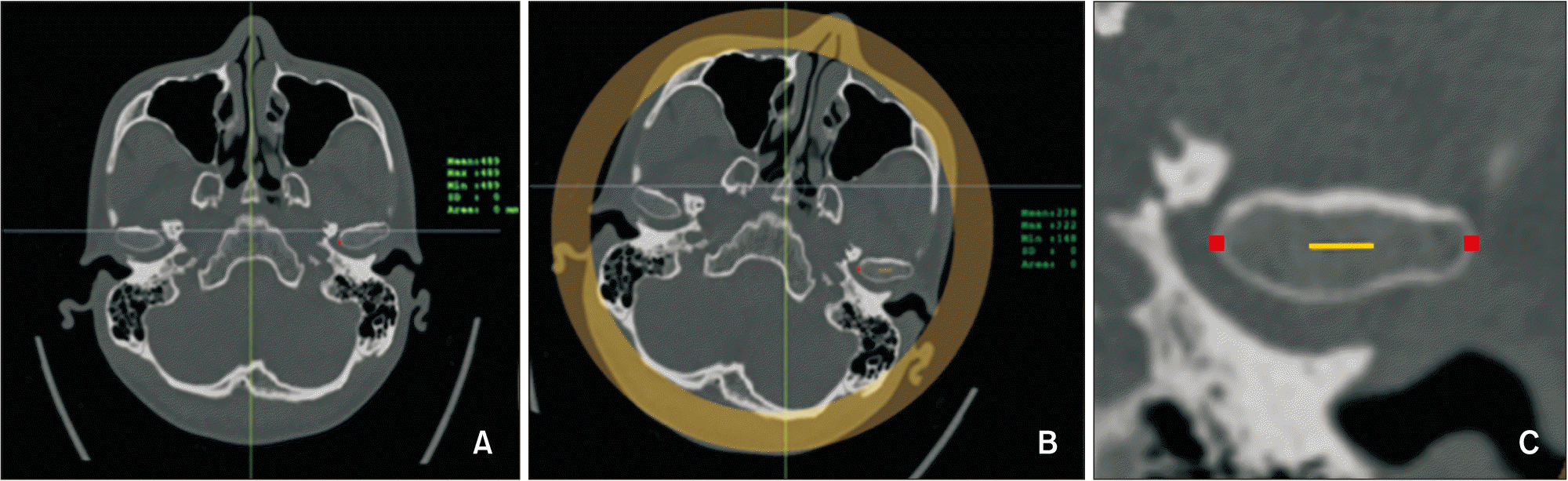 | Figure 6Bone density measurement at the condyle. A, The axial slice with the largest mediolateral diameter of the mandibular condyle was selected. B, The condyle was re-oriented as the most medial and lateral points aligned in a horizontal line. C, Cortical bone densities at the medial and lateral points were measured (red dots), and cancellous bone density was measured in the middle 5 mm upon the line connecting the medial and lateral points. |
 | Figure 7Bone density measurements for the fourth cervical vertebrae (C4). A, Orientation of computed tomography images for C4. B, Measurement sites for C4 (yellow arrow). C, Magnified view of C4. Cortical bone densities were measured at the most anterosuperior and posteroinferior red points and cancellous bone density was measured in the middle 5 mm of the line (yellow) connecting the two red points. |
Statistical analysis
RESULTS
Table 2
| Site | T1 | T2 | Δ (T2–T1) | p-value | Number | ||
|---|---|---|---|---|---|---|---|
| Tuberosity | 5 mm | BC | 763.0 ± 138.4 | 746.8 ± 102.2 | −16.2 ± 80.7 | 0.677 | 5 |
| LC | 919.8 ± 117.8 | 892.2 ± 114.3 | −27.6 ± 45.6 | 0.247 | 5 | ||
| Can | 212.2 ± 66.9 | 120.0 ±87.3 | −92.2 ± 57.4 | 0.023* | 5 | ||
| 10 mm | BC† | 1,024.5 ± 277.0 | 1,034.5 ± 234.2 | 10.0± 107.2 | > 0.999 | 4 | |
| LC | 1,285.5 ± 215.8 | 1,215.5 ± 206.6 | −70.0 ± 11.9 | 0.001** | 4 | ||
| Can | - | - | - | - | |||
| 15 mm | BC | - | - | - | - | 0 | |
| LC | - | - | - | - | 0 | ||
| Can | - | - | - | - | 0 | ||
| Molar | 5 mm | BC | 1,059.2 ± 138.1 | 1,053.2 ± 138.3 | −6.0 ± 37.9 | 0.648 | 9 |
| LC | 1,198.0 ± 295.7 | 1,168.2 ± 282.8 | −29.8 ± 65.1 | 0.185 | 10 | ||
| Can | 452.5 ± 247.0 | 394.4 ± 190.3 | −58.1 ± 71.7 | 0.055 | 8 | ||
| 10 mm | BC | 1,243.1 ± 346.9 | 1,154.4 ± 310.9 | −88.8 ± 133.7 | 0.103 | 8 | |
| LC | 1,407.8 ± 249.5 | 1,392.1 ± 172.5 | −15.7 ± 131.1 | 0.714 | 10 | ||
| Can | 204.5 ± 316.1 | 115.0 ± 87.7 | −89.5 ± 228.4 | 0.678 | 2 | ||
| 15 mm | BC† | 1,540.0 ± 156.1 | 1,460.5 ± 109.6 | −79.5 ± 72.5 | 0.109 | 4 | |
| LC | 1,110.4 ± 179.9 | 1,113.0 ± 193.5 | 2.6 ± 78.7 | 0.945 | 5 | ||
| Can | 174.0 | 277.0 | 103.0 | - | 1 | ||
| Premolar | 5 mm | BC | 1,225.7 ± 181.3 | 1,222.0 ± 261.7 | −3.7 ± 126.9 | 0.929 | 10 |
| LC | 1,246.1 ± 163.1 | 1,274.6 ± 178.5 | 28.5 ± 83.0 | 0.306 | 10 | ||
| Can | 464.7 ± 271.2 | 372.4 ± 251.0 | −92.3 ± 94.4 | 0.013* | 10 | ||
| 10 mm | BC† | 1,297.5 ± 387.9 | 1,271.8 ± 364.1 | −25.7 ± 64.1 | 0.203 | 10 | |
| LC | 1,353.1 ± 203.7 | 1,377.2 ± 219.8 | 24.1 ± 117.0 | 0.531 | 10 | ||
| Can | 380.6 ± 261.4 | 292.4 ± 236.0 | −88.1 ± 55.7 | 0.006** | 7 | ||
| 15 mm | BC† | 1,639.6 ± 201.3 | 1,589.9 ± 174.5 | −49.8 ± 116.7 | 0.263 | 8 | |
| LC | 1,220.4 ± 319.8 | 1,197.9 ± 355.2 | −22.6 ± 75.4 | 0.458 | 7 | ||
| Can† | 220.5 ± 294.9 | 218.0 ± 253.1 | −2.5 ± 41.7 | 0.655 | 2 | ||
| Incisal | 5 mm | BC† | 1,101.3 ± 335.8 | 1,061.5 ± 286.6 | −39.8 ± 92.9 | 0.093 | 10 |
| LC† | 1,068.1 ± 268.4 | 1,040.1 ± 258.0 | −28.0 ± 154.1 | 0.285 | 10 | ||
| Can | 568.8 ± 292.4 | 452.8 ± 255.2 | −116.0 ± 135.7 | 0.046* | 8 | ||
| 10 mm | BC | 1,043.6 ± 286.4 | 1,044.2 ± 324.3 | 0.6 ± 120.6 | 0.988 | 10 | |
| LC† | 1,303.1 ± 177.0 | 1,271.7 ± 207.9 | −31.4 ± 142.3 | 0.333 | 10 | ||
| Can | 498.6 ± 201.7 | 416.2 ± 224.1 | −82.3 ± 119.7 | 0.073 | 9 | ||
| 15 mm | BC† | 1,360.1 ± 215.1 | 1,309.9 ± 272.6 | −50.2 ± 70.0 | 0.059 | 10 | |
| LC | 1,308.4 ± 165.3 | 1,273.0 ± 152.5 | −35.4 ± 67.7 | 0.132 | 10 | ||
| Can | 235.6 ± 120.1 | 195.1 ± 112.5 | −40.4 ± 39.9 | 0.016* | 9 | ||
Values are presented as mean ± standard deviation or number only when only one measurement was possible.
Some measurements could not be obtained due to adjacent anatomic structures (i.e., third molar, maxillary sinus) or other objects (i.e., orthodontic miniscrew).
Table 3
| Site | T1 | T2 | Δ (T2–T1) | p-value | Number | ||
|---|---|---|---|---|---|---|---|
| Retromolar pad | 5 mm | BC† | 1,695.6 ± 337.5 | 1,640.5 ± 269.0 | −55.1 ± 94.0 | 0.093 | 10 |
| LC | 1,196.6 ± 361.7 | 1,145.7 ± 329.9 | −50.9 ± 38.4 | 0.002** | 10 | ||
| Can | 219.1 ± 130.9 | 150.8 ± 105.5 | −68.3 ± 91.1 | 0.042* | 10 | ||
| 10 mm | BC | 1,904.1 ± 93.8 | 1,824.8 ± 146.7 | −79.3 ± 129.2 | 0.084 | 10 | |
| LC | 1,759.1 ± 74.3 | 1,661.3 ± 62.0 | −97.8 ± 110.7 | 0.021* | 10 | ||
| Can | 245.9 ± 127.6 | 175.4 ± 103.4 | −70.5 ± 57.6 | 0.004** | 10 | ||
| 15 mm | BC | 1,919.8 ± 102.4 | 1,904.3 ± 105.7 | −15.5 ± 32.3 | 0.163 | 10 | |
| LC | 1,723.8 ± 102.6 | 1,700.0 ± 100.9 | −23.8 ± 84.3 | 0.395 | 10 | ||
| Can | 159.4 ± 99.3 | 102.3 ± 109.4 | −57.1 ± 62.4 | 0.018* | 10 | ||
| Molar | 5 mm | BC | 1,575.5 ± 95.9 | 1,541.6 ± 121.9 | −33.9 ± 70.9 | 0.165 | 10 |
| LC | 1,660.9 ± 123.1 | 1,577.8 ± 113.3 | −83.1 ± 65.9 | 0.003** | 10 | ||
| Can | 548.9 ± 172.4 | 449.7 ± 147.4 | −99.2 ± 72.2 | 0.002** | 10 | ||
| 10 mm | BC | 1,829.2 ± 103.6 | 1,749.2 ± 166.2 | −80.0 ± 86.2 | 0.017* | 10 | |
| LC | 1,800.1 ± 88.0 | 1,750.3 ± 127.3 | −49.8 ± 80.4 | 0.082 | 10 | ||
| Can | 188.7 ± 156.3 | 110.9 ± 149.0 | −77.8 ± 72.9 | 0.008** | 10 | ||
| 15 mm | BC | 1,886.8 ± 89.7 | 1,820.7 ± 102.9 | −66.1 ± 105.6 | 0.079 | 10 | |
| LC | 1,796.2 ± 121.6 | 1,757.6 ± 103.9 | −38.6 ± 61.5 | 0.078 | 10 | ||
| Can | 111.9 ± 157.0 | 71.4 ± 136.8 | −40.5 ±30.6 | 0.002** | 10 | ||
| Premolar | 5 mm | BC | 1,435.5 ± 148.6 | 1,369.2 ± 131.7 | −66.3 ± 53.7 | 0.004** | 10 |
| LC | 1,589.5 ± 112.8 | 1,505.9 ± 143.3 | −83.6 ± 88.7 | 0.015* | 10 | ||
| Can | 183.9 ± 142.8 | 132.4 ± 123.1 | −51.5 ± 47.4 | 0.007** | 10 | ||
| 10 mm | BC | 1,573.2 ± 175.6 | 1,544.2 ± 118.0 | −29.0 ± 167.6 | 0.598 | 10 | |
| LC | 1,782.5 ± 115.6 | 1,700.3 ± 107.0 | −82.2 ± 105.1 | 0.035* | 10 | ||
| Can† | 211.9 ± 136.3 | 137.2 ± 135.0 | −74.7 ± 88.1 | 0.009** | 10 | ||
| 15 mm | BC | 1,852.0 ± 139.8 | 1,758.9 ± 133.2 | −83.1 ± 101.7 | 0.030* | 10 | |
| LC | 1,883.1 ± 106.4 | 1,804.8 ± 121.5 | −78.3 ± 99.3 | 0.034* | 10 | ||
| Can | 156.5 ± 185.2 | 89.8 ± 150.9 | −66.7 ± 50.3 | 0.002** | 10 | ||
| Incisal | 5 mm | BC | 764.6 ± 208.1 | 713.5 ± 214.9 | −51.1 ± 55.9 | 0.018* | 10 |
| LC | 1,051.4 ± 159.6 | 984.7 ± 204.4 | −66.7 ± 90.1 | 0.044* | 10 | ||
| Can | 510.0 ± 204.5 | 407.3 ± 219.2 | −102.7 ±73.5 | 0.002** | 10 | ||
| 10 mm | BC | 1,142.0 ± 358.7 | 1,129.6 ± 383.9 | −12.4 ± 51.6 | 0.467 | 10 | |
| LC† | 1,464.7 ± 253.3 | 1,446.3 ± 264.7 | −18.4 ± 37.8 | 0.241 | 10 | ||
| Can | 662.3 ± 204.3 | 572.8 ± 212.7 | −89.6 ± 34.5 | < 0.001*** | 9 | ||
| 15 mm | BC | 1,632.3 ± 259.6 | 1,596.3 ± 241.6 | −36.0 ± 69.0 | 0.133 | 10 | |
| LC | 1,881.5 ± 134.9 | 1,793.9 ± 197.1 | −87.6 ± 113.0 | 0.037* | 10 | ||
| Can | 517.2 ± 240.0 | 423.0 ± 172.6 | −94.2 ± 88.5 | 0.013* | 9 | ||
Table 4
| Site | T1 | T2 | Δ (T2–T1) | p-value | |
|---|---|---|---|---|---|
| Menton | 1,888.5 ± 141.4 | 1,843.7 ± 139.9 | −44.8 ± 48.7 | 0.017* | |
| Top of head | 1,730.2 ± 118.5 | 1,698.6 ± 122.0 | −31.6 ± 85.0 | 0.270 | |
| Condyle | Medial cortex | 872.7 ± 269.3 | 879.3 ± 293.1 | 6.6 ± 99.4 | 0.838 |
| Lateral cortex | 887.9 ± 233.6 | 870.4 ± 295.5 | −17.5 ± 120.0 | 0.656 | |
| Can | 230.9 ± 69.6 | 204.1 ± 72.3 | −26.8 ± 38.6 | 0.056 | |
| Vertebrae | ASC | 736.9 ± 306.4 | 766.3 ± 265.7 | 29.4 ± 150.8 | 0.553 |
| PIC | 1,068.9 ± 298.9 | 1,050.3 ± 318.9 | −18.6 ± 129.8 | 0.661 | |
| DCan | 371.9 ± 94.3 | 317.7 ± 122.6 | −54.2 ± 52.6 | 0.010* | |
Table 5
Table 6
| Site | With G (n = 6) | Without G (n = 4) | p-value | ||
|---|---|---|---|---|---|
| Tuberosity | 5 mm | BC† | −11.5 ± 78.5 | −19.3 ± 99.6 | > 0.999 |
| LC† | −25.0 ± 83.4 | −29.3 ± 25.7 | > 0.999 | ||
| Can† | −66.5 ± 33.2 | −109.3 ± 70.3 | 0.400 | ||
| 10 mm | BC† | −62.5 ± 33.2 | 82.5 ± 111.0 | 0.333 | |
| LC† | −69.0 ± 5.7 | −71.0 ± 19.8 | > 0.999 | ||
| Can | - | - | - | ||
| 15 mm | BC | - | - | - | |
| LC | - | - | - | ||
| Can | - | - | - | ||
| Molar | 5 mm | BC | −31.0 ± 39.7 | 6.5 ± 33.3 | 0.176 |
| LC | −8.3 ± 69.2 | −44.2 ± 64.2 | 0.425 | ||
| Can | −46.3 ± 81.5 | −70.0 ± 70.5 | 0.675 | ||
| 10 mm | BC | 2.0 ± 35.8 | −179.5 ± 135.9 | 0.042* | |
| LC | −32.8 ± 64.7 | −4.3 ± 167.4 | 0.758 | ||
| Can† | 72.0 | −251.0 | > 0.999 | ||
| 15 mm | BC† | −45.0 ± 63.6 | −114.0 ± 83.4 | 0.667 | |
| LC† | 23.5 ± 33.2 | −11.3 ± 105.4 | > 0.999 | ||
| Can | 103.0 | - | - | ||
| Premolar | 5 mm | BC | −119.5 ± 83.6 | 73.5 ± 83.2 | 0.007** |
| LC | −34.8 ± 51.2 | 70.7 ± 74.2 | 0.040* | ||
| Can† | −71.8 ± 32.0 | −106.0 ± 121.9 | 0.064 | ||
| 10 mm | BC | −19.8 ± 70.7 | −29.7 ± 66.0 | 0.826 | |
| LC | 3.5 ± 19.8 | 37.8 ± 154.4 | 0.676 | ||
| Can | −112.3 ± 63.1 | −56.0 ± 24.6 | 0.211 | ||
| 15 mm | BC | −64.8 ± 166.2 | −34.8 ± 59.5 | 0.746 | |
| LC | −50.7 ± 99.1 | −1.5 ± 58.7 | 0.444 | ||
| Can | −2.5 ± 41.7 | - | - | ||
| Incisal | 5 mm | BC | −4.5 ± 130.6 | −63.3 ± 60.2 | 0.356 |
| LC | −147.5 ± 61.7 | 51.7 ± 146.4 | 0.035* | ||
| Can | −176.3 ± 88.4 | −79.8 ± 154.7 | 0.369 | ||
| 10 mm | BC | −64.3 ± 81.4 | 43.8 ± 128.8 | 0.178 | |
| LC | −57.5 ± 90.6 | −14.0 ± 175.0 | 0.663 | ||
| Can | −122.3 ± 95.7 | −62.3 ± 133.4 | 0.515 | ||
| 15 mm | BC | −99.0 ± 75.1 | −17.7 ± 47.5 | 0.066 | |
| LC | −22.0 ± 15.4 | −44.3 ± 88.6 | 0.638 | ||
| Can | −12.3 ± 35.2 | −54.5 ± 36.5 | 0.143 | ||
Values are presented as mean ± standard deviation or number only when only one measurement was possible.
Some measurements could not be obtained due to adjacent anatomic structures (i.e., third molar, maxillary sinus) or other objects (i.e., orthodontic miniscrew).
Table 7
| Site | With G (n = 6) | Without G (n = 4) | p-value | ||
|---|---|---|---|---|---|
| Retromolar pad | 5 mm | BC | −33.0 ± 77.4 | −69.8 ± 107.9 | 0.575 |
| LC† | −35.5 ± 20.7 | −61.2 ± 45.6 | 0.114 | ||
| Can | 5.3 ± 80.9 | −117.3 ± 61.8 | 0.026* | ||
| 10 mm | BC | −108.8 ± 149.7 | −59.7 ± 124.2 | 0.587 | |
| LC | −91.0 ± 80.0 | −102.3 ± 134.7 | 0.885 | ||
| Can | −52.8 ± 41.1 | −82.3 ± 67.3 | 0.459 | ||
| 15 mm | BC | −11.0 ± 13.9 | −18.5 ± 41.6 | 0.696 | |
| LC† | −13.5 ± 22.0 | −30.7 ± 111.2 | 0.610 | ||
| Can | −36.8 ± 35.7 | −70.7 ± 75.4 | 0.432 | ||
| Molar | 5 mm | BC | −48.0 ± 45.0 | −24.5 ± 87.1 | 0.637 |
| LC | −72.8 ± 75.3 | −90.0 ± 65.3 | 0.710 | ||
| Can | −141.8 ± 89.8 | −70.8 ± 46.2 | 0.135 | ||
| 10 mm | BC | −95.0 ± 119.3 | −70.0 ± 67.4 | 0.680 | |
| LC† | −70.3 ± 117.5 | −36.2 ± 52.9 | > 0.999 | ||
| Can† | −80.8 ± 84.6 | −75.8 ± 72.4 | > 0.999 | ||
| 15 mm | BC | −105.5 ± 159.5 | −39.8 ± 52.3 | 0.366 | |
| LC | −36.0 ± 47.5 | −40.3 ± 73.8 | 0.920 | ||
| Can | −52.8 ± 21.6 | −32.3 ± 34.7 | 0.285 | ||
| Premolar | 5 mm | BC | −68.5 ± 68.5 | −64.8 ± 48.7 | 0.923 |
| LC | −74.8 ± 70.2 | −89.5 ± 105.3 | 0.813 | ||
| Can | −23.0 ± 16.2 | −70.5 ± 53.0 | 0.126 | ||
| 10 mm | BC | −41.5 ± 80.2 | −20.7 ± 215.6 | 0.860 | |
| LC† | −87.0 ± 70.9 | −79.0 ± 129.8 | 0.610 | ||
| Can | −24.8 ± 32.9 | −108.0 ± 99.9 | 0.104 | ||
| 15 mm | BC | −122.5 ± 146.6 | −56.8 ± 60.6 | 0.347 | |
| LC | −133.3 ± 146.8 | −41.7 ± 28.2 | 0.302 | ||
| Can | −57.5 ± 33.8 | −72.8 ± 61.3 | 0.664 | ||
| Incisal | 5 mm | BC | −67.0 ± 50.0 | −40.5 ± 61.6 | 0.496 |
| LC | −72.8 ± 88.1 | −62.7 ± 99.6 | 0.874 | ||
| Can | −142.3 ± 96.2 | −76.3 ± 45.6 | 0.177 | ||
| 10 mm | BC | −41.5 ± 38.6 | 7.0 ± 52.6 | 0.155 | |
| LC | −21.0 ± 33.4 | −16.7 ± 43.5 | 0.871 | ||
| Can† | −94.7 ± 27.4 | −87.0 ± 39.8 | 0.548 | ||
| 15 mm | BC | −53.5 ± 111.6 | −24.3 ± 26.2 | 0.641 | |
| LC | −180.8 ± 111.8 | −25.5 ± 62.6 | 0.022* | ||
| Can | −155.0 ± 132.9 | −63.8 ± 46.2 | 0.156 | ||
Table 8
| Site | With G (n = 6) | Without G (n = 4) | p-value | |
|---|---|---|---|---|
| Menton | −75.3 ± 67.5 | −24.5 ± 17.2 | 0.230 | |
| Top of head | −97.0 ± 99.6 | 12.0 ± 36.6 | 0.037* | |
| Condyle | Medial cortex† | −37.5 ± 55.9 | 36.0 ± 115.4 | 0.352 |
| Lateral cortex† | −56.5 ± 49.8 | 8.5 ± 149.7 | 0.476 | |
| Can | −58.3 ± 39.1 | −5.8 ± 21.2 | 0.024* | |
| Vertebrae | ASC | 5.8 ± 84.2 | 45.2 ± 189.5 | 0.710 |
| PIC | −65.5 ± 72.8 | 12.7 ± 155.5 | 0.382 | |
| DCan† | −83.5 ± 49.7 | −34.7 ± 48.5 | 0.114 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download