INTRODUCTION
Preservation of the vital periodontal ligament (PDL) on a donor tooth is a key factor for successful tooth autotransplantation (TAT).
1 The PDL attached to the root surface contains several cell types essential for the prevention of root resorption.
2 PDL injuries often occur during the extraction of donor teeth, and this leads to root resorption and ankylosis, which result in unsuccessful TAT.
3 The two main tissues involved in TAT healing are the PDL and alveolar bone. The healing of the PDL after TAT involves proliferative fibroblasts that are the most prevalent cell type within the PDL responsible for the production and alignment of collagen fiber bundles; in contrast, healing of the alveolar bone involves the bone remodeling process that begins with bone resorption by osteoclasts coupled with bone formation by osteoblasts.
2 In addition to the synthesis and organization of collagen fibers, the proliferative PDL fibroblasts secrete various bone mediators that control osteoblast and osteoclast differentiation in response to mechanical stresses.
4
Several bone mediators have been studied during orthodontic tooth movement, and their levels vary in the PDL of animals and humans.
5-8 Among these mediators, runt-related transcription factor 2 (RUNX2)
5,9 and alkaline phosphatase (ALP)
9-11 are commonly used to monitor osteoblast differentiation under mechanical stresses. RUNX2 is a specific transcription factor that activates mesenchymal cells of the bone marrow, leading to their differentiation into osteoblasts. Additionally, this transcription factor can regulate osteoblast maturation.
6 ALP is commonly used to assess the phenotypic development of osteoblasts,
12 and its expression reflects an early stage of osteoblastic differentiation.
11 Osteoblasts also directly regulate osteoclast activity during bone remodeling.
10 Osteoblastic lineage cells express receptor activator of nuclear factor kappa-B ligand (RANKL),
13 which is a key factor mediating osteoclastogenesis and is considered an osteoclastic biomarker.
10 RANKL triggers osteoclast formation and activity once it binds to its specific receptor, called RANK, on the osteoclast precursor cell membrane. To exert further control, osteoblasts produce a potent inhibitor of RANKL, i.e., osteoprotegerin (OPG),
14 which is a member of the tumor necrosis factor receptor family. OPG functions by binding to RANKL and interrupting the RANKL-RANK interaction, thereby resulting in the inhibition of osteoclastogenesis.
13
In a few previous studies, orthodontic force application before TAT resulted in increased PDL volume.
15 Consequently, such force application is recommended to enhance the success rate of transplantation.
16 However, the expressions of bone biomolecules within the PDL at different loading durations and the optimal preloading duration to obtain the greatest PDL volume remain unclear. Therefore, the aims of this study were to evaluate the optimal orthodontic preloading duration to increase PDL volume and to determine the expressions of the bone biomolecules RUNX2, ALP, RANKL, and OPG in human PDL tissue upon loading with a leveling force at different time points.
Go to :

MATERIALS AND METHODS
Patient selection
Healthy, non-smoking patients requiring extraction of the first premolars for orthodontic treatment were recruited from the Postgraduate Clinic, Faculty of Dentistry, Bangkokthonburi University, Thailand, after obtaining written informed consent. The study protocol (#9/2018) was approved by the Bangkokthonburi University Human Ethics Committee. The inclusion criteria were as follows: patients who had sound first premolars with complete root formation in their four quadrants without caries or restorations, and had good oral hygiene and a healthy periodontium. The exclusion criteria were as follows: patients with systemic infections or diseases who required medications, such as nonsteroidal inflammatory drugs, or those with severe crowding at the extraction sites.
Eighteen such patients undergoing orthodontic treatment were randomly divided into two groups for the measurement of PDL volume and determination of protein expression. For the measurement of PDL volume, 32 first premolars from eight patients (mean age, 21.0 ± 3.8 years; six females and two males) were selected. The sample size of at least eight teeth for each period of loading was determined using G*Power software ver. 3.1.9.2 (Franz Faul; University of Kiel, Kiel, Germany), with an effect size = 1.45 derived from the preliminary data, α = 0.05, and 1-β = 0.95. To determine protein expression, 40 first premolars from the ten remaining patients (mean age, 21.4 ± 4.0 years; eight females and two males) were selected. The sample size of at least nine teeth for each period of loading was determined with an effect size = 1.33 derived from the preliminary data, α = 0.05, and 1-β = 0.95.
Orthodontic preloading and tooth extraction
The orthodontic leveling force was applied using a 0.016-inch continuous archwire, which is an improved superelastic nickel-titanium alloy wire (Sentalloy blue, Sentalloy
®; Tomy International Inc., Tokyo, Japan), inserted into a preadjusted edgewise bracket (Roth prescription; 0.018 × 0.025 inch, Tomy International Inc.) that was bonded on the first premolar and all remaining teeth. The bracket was attached using an indirect bonding technique to allow precise bracket positioning.
17 For the first premolars, the brackets were passively placed to avoid either intrusive or extrusive movements. The wire possesses a shape-memory effect, which enables the generation of a light continuous force at 30–70 g in the oral cavity.
18 The control unloaded premolars were extracted at each patient’s first visit, whereas the experimental loaded premolars were extracted after orthodontic loading for 1, 2, or 4 weeks. Simple exodontia was performed by one oral surgeon, with the patients under local anesthesia induced using 4% articaine and 1:100,000 epinephrine (Septanest SP
®; Septodont Co., Paris, France) administered via an infiltration technique (1.0 mL for the maxillary premolars) or an inferior alveolar nerve block together with lingual and long buccal nerve blocks (1.7 mL for the mandibular premolars). A conventional aspirating dental syringe and a disposable 30-gauge and 21-mm-long needle (Septoject XL
®; Septodont Co.) were used for the infiltration, and a disposable 27-gauge and 30-mm-long needle (Septoject XL
®; Septodont Co.) were used for the nerve blocks. Both experimental and control premolars were atraumatically extracted by gentle separation of the gingiva using a straight elevator no. EL3S (Hu-Friedy Mfg. Co., LLC, Chicago, IL, USA), followed by the use of a no. 151 forceps (Hu-Friedy Presidential
®; Hu-Friedy Mfg. Co., LLC) to avoid injury to the PDL tissue. The extracted premolars were then washed with normal saline to remove blood and tissue debris.
Assessment of periodontal ligament tissue
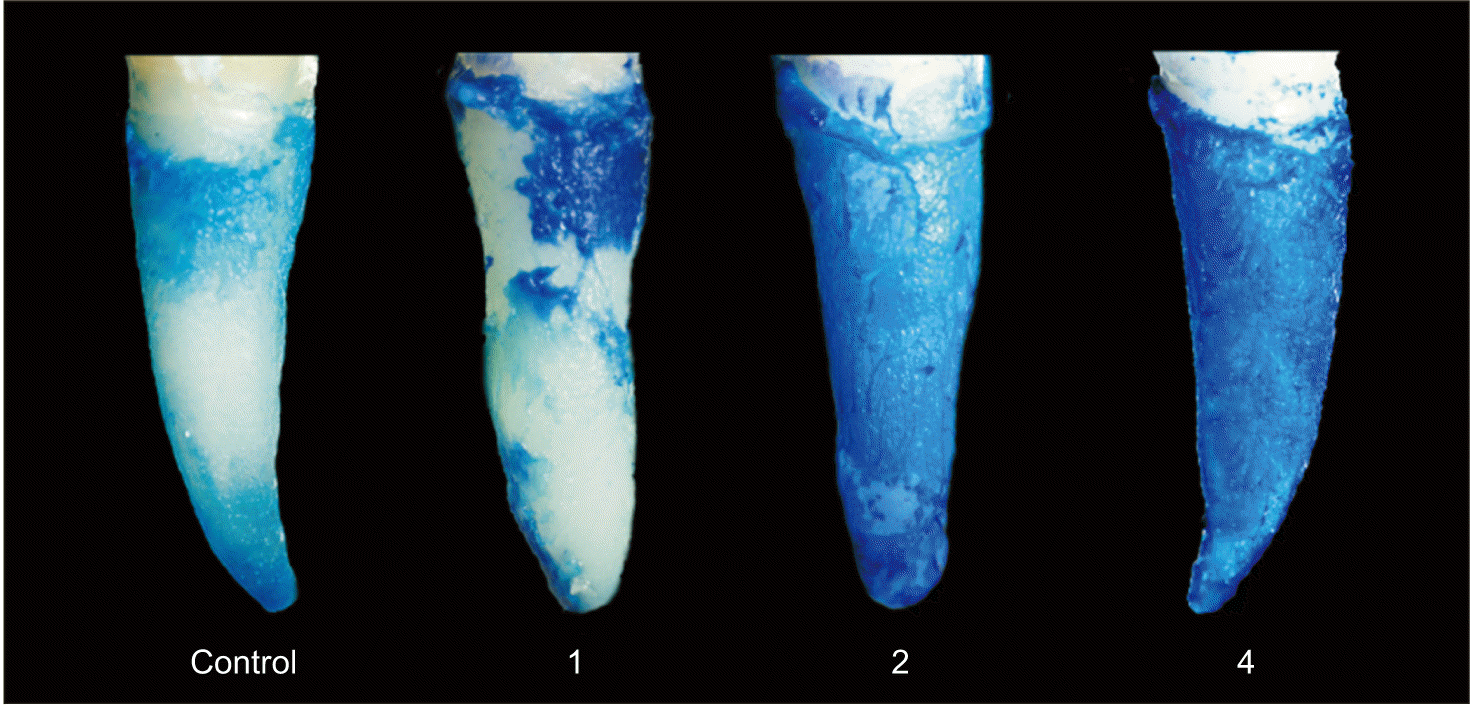
Toluidine blue staining and determination of the remaining PDL volume on the root surface were performed using the protocol of Nakdilok et al.
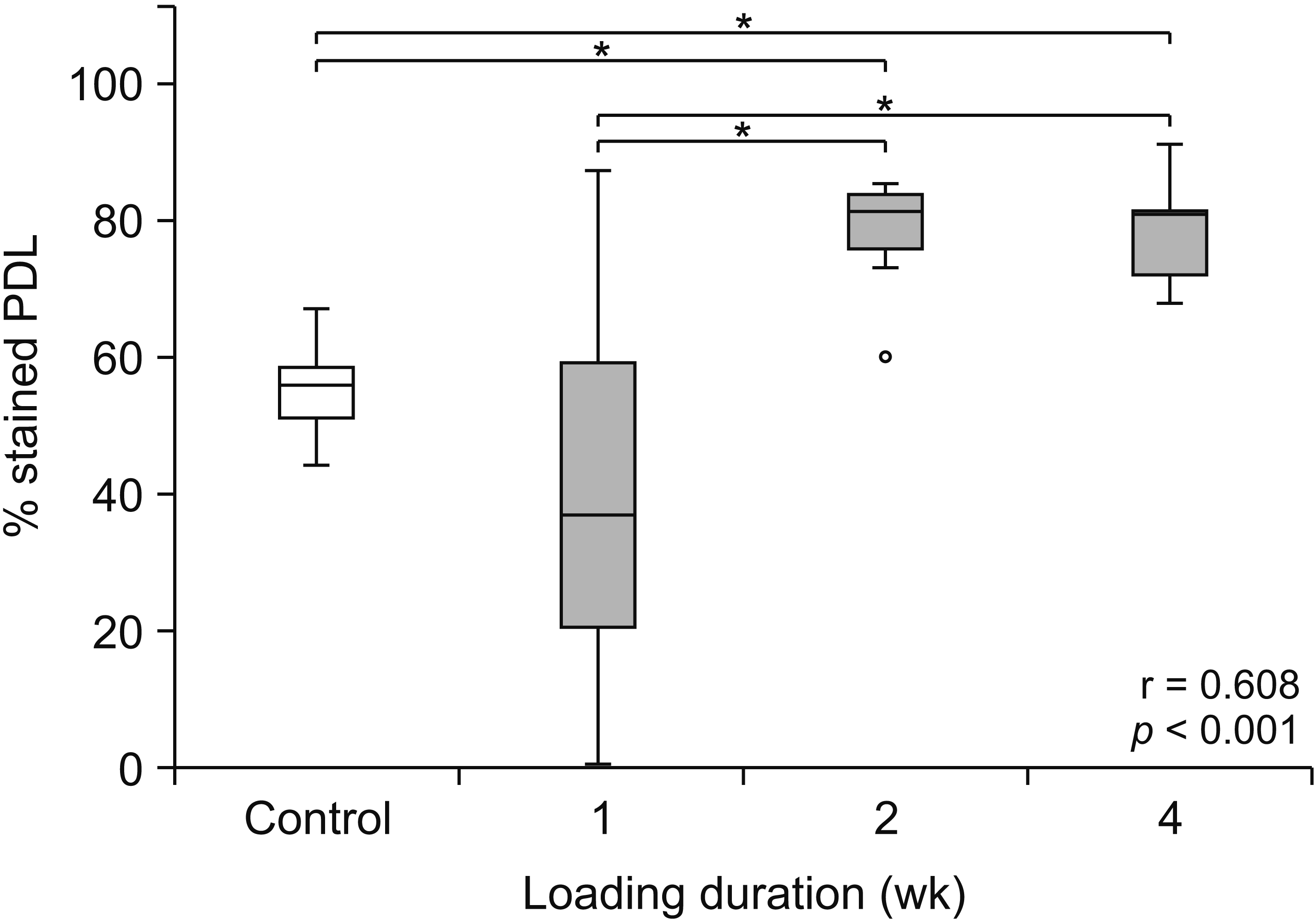
19 Briefly, the extracted teeth were stained with 0.04% (w/v) toluidine blue (Sigma-Aldrich, St. Louis, MO, USA) and destained with phosphate buffered saline for 14 days. A stereomicroscope (Olympus SZX7; Olympus Corp., Tokyo, Japan) was used to observe all surfaces of the stained roots. A digitized image in a plane perpendicular to the tooth axis was then recorded using a charge-coupled device (Olympus E-330; Olympus Corp.) attached to the stereomicroscope. The ImageJ2 software (National Institutes of Health, Bethesda, MD, USA) was used to quantify the stained images, and the stained area in each image was determined as a percentage of the total area. The percentage of stained PDL was calculated using ImageJ2 by differentiating between the white and blue pixels, which represented the unstained and stained areas, respectively. The percentage of stained PDL of each tooth was then calculated.
Expressions of bone biomolecules in periodontal ligament tissue
The PDL tissue of the extracted premolars was scraped off from 3 mm below the cementoenamel junction to the root apex. Total protein was isolated by grinding the PDL tissue in a glass homogenizer using 350 μL of lysis buffer from a NucleoSpin
® RNA/Protein isolation kit (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany) and 1% (v/v) β-mercaptoethanol (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The lysate was transferred to a 1.5-mL microcentrifuge tube and stored at –80°C until simultaneous western blot analysis of every sample from the ten patients. The frozen lysates were thawed and their total protein was extracted using the NucleoSpin
® RNA/Protein isolation kit (MACHEREY-NAGEL GmbH & Co. KG) following the manufacturer’s instruction. In brief, the lysates were first centrifuged at 1,100 ×
g for 1 minute to remove tissue debris by using filter tubes, and the filtrated lysates were mixed with 70% ethanol at an equal volume. The mixtures were centrifuged using the NucleoSpin
® columns to collect their protein fraction from the flow-through solution. Protein in the solution was precipitated and dissolved in a protein solving buffer containing the reducing agent Tris (2-carboxyethyl) phosphine hydrochloride. The quantity of total protein in each sample was determined using ultraviolet absorbance at 280 nm in a NanoDrop
TM 2000 spectrophotometer (Thermo Scientific
TM, Rockford, IL, USA). A 20-μg quantity of total protein was resolved using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis under an electrical current at 100 V/300 W for 135 minutes, and was transferred to nitrocellulose membranes under an electrical current at 100 mA/300 W for 12 hours. To block non-specific binding, the membranes were incubated for 1 hour in 5% (w/v) non-fat dry milk (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) in 0.1% (v/v) Tween-20/Tris-buffered saline. Subsequently, the membranes were incubated overnight at 4°C with the primary antibodies. On the following day, the membranes were washed and incubated with the secondary antibodies for 1 hour. The primary and secondary antibodies used in this study are summarized in
Table 1. After washing, the membranes were reacted with LumiGLO Reserve
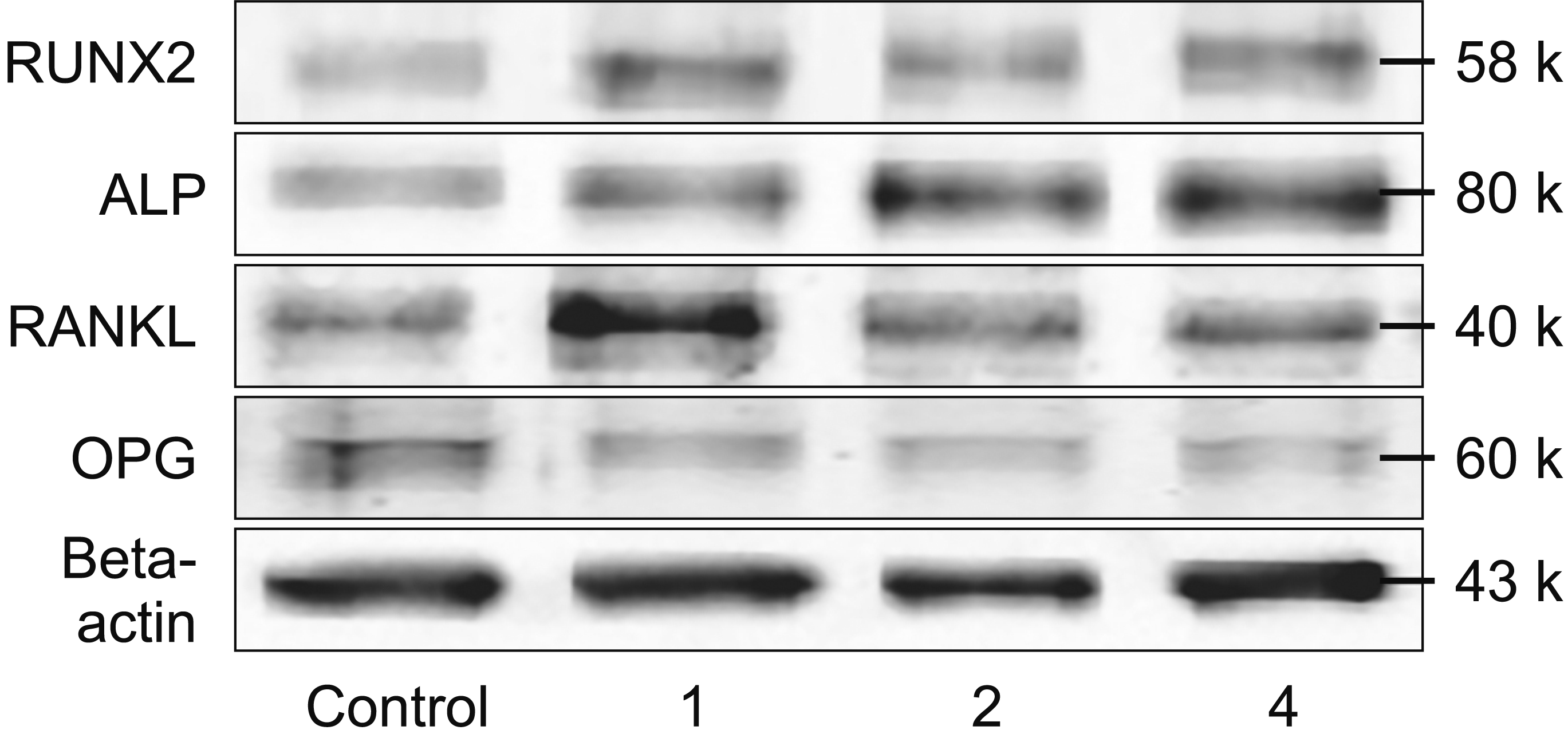
TM Chemiluminescent reagent (KPL, Gaithersburg, MD, USA). Chemiluminescent signals were captured using the ChemiDoc XRS gel documentation system (Bio-Rad Laboratories, Inc.). The intensities of RUNX2, ALP, RANKL, and OPG bands at their predicted size were measured using ImageJ2 and normalized by that of the beta actin band in each sample. Thereafter, the normalized intensities of these proteins were compared between the experimental and control groups. In addition, the relative ratios of normalized RANKL to OPG were determined and compared between the experimental and control groups.
Table 1
|
Antibody |
Donor |
Dilution |
Supplier |
|
Primary antibody |
|
Polyclonal IgG anti-human RUNX2/CBFA1 |
Goat |
1:2,000 |
R&D systems, Minneapolis, MN, USA |
|
Monoclonal IgG1 anti-human ALPL |
Mouse |
1:2,000 |
R&D systems |
|
Monoclonal IgG1 anti-human RANKL |
Mouse |
1:500 |
Santa Cruz Biotechnology, Santa Cruz, CA, USA |
|
Monoclonal IgG1 anti-human OPG |
Mouse |
1:500 |
Santa Cruz Biotechnology |
|
Monoclonal IgG1 anti-human beta-actin |
Mouse |
1:1,000 |
Santa Cruz Biotechnology |
|
Secondary antibody |
|
Anti-rabbit immunoglobulins |
Horseradish peroxidase (HRP)-conjugated swine |
1:2,000 |
Dako, Glostrup, Denmark |
|
Anti-mouse immunoglobulins |
HRP-conjugated rabbit |
1:2,000 |
R&D systems |
|
Anti-goat IgG (H + L) |
HRP-mouse |
1:2,000 |
Thermo Scientific, Rockford, IL, USA |

Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., Armonk, NY, USA). Comparisons between the percentages of overall stained PDL tissue at different time points, significant differences between protein expressions, and the RANKL/OPG ratios at different orthodontic loading durations were tested using the Friedman test, followed by the Wilcoxon signed-rank test. The differences were considered statistically significant if p-values were less than 0.05. Spearman’s method was used to analyze correlations between the percentages of stained PDL, protein expressions, or the RANKL/OPG ratios and increased loading durations.
Go to :

DISCUSSION
PDL is a fiber-reinforced tissue containing various types of cells that play important roles in the response to mechanical stresses, maintaining the periodontium, and promoting periodontal regeneration.
9 The results of this study demonstrated that orthodontic preloading led to macroscopic and molecular alterations in the PDL tissue. During the early stage of this process, i.e., after the application of the leveling force for 1 week, no significant changes were observed in the percentages of stained PDL, whereas significant increases in RUNX2 expression and the RANKL/OPG ratio were noted. Two weeks after orthodontic preloading, PDL volume and the expression of ALP were significantly increased, and the degree of expression of RUNX2 remained high. At the end of the observation, i.e., after 4 weeks of preloading with the leveling force, PDL volume and the expressions of RUNX2 and ALP along with the RANKL/OPG ratio were approximately at their maximal levels and were greater in the loaded teeth than in the unloaded teeth. Taken together, the preloading duration appears to be a crucial factor that increases proliferative PDL volumes, as shown by an increase in the percentage of stained PDL, the expressions of RUNX2 and ALP, as well as the RANKL/OPG ratio within the PDL.
Preapplication of orthodontic loading for various durations has been previously performed in several studies. In an animal study, the preloading of a light continuous orthodontic force for 7 days resulted in rich PDL tissue attached to the extracted teeth.
15 A study performed in humans also demonstrated that preoperative application of an extrusion force for 2 to 3 weeks helped prevent extraction failure and increased the success rate of tooth replantation.
16 Moreover, our previous study showed a significant increase in PDL volume and facilitated tooth extraction upon orthodontic preloading for 4 weeks.
19 However, the time-course study of orthodontic preloading that may help increase the success rate of TAT has not been discussed.
Our study suggests that increased proliferative PDL and the expression profiles of the four bone biomolecules derived from the proliferative PDL are associated with various clinical phases of orthodontic tooth movement. In the initial phase, PDL becomes disorganized; the collagen fiber orientation in the PDL is considerably changed by the function of PDL fibroblasts.
20 These cells play crucial roles in synthesizing new collagen fibers and ground substances while simultaneously breaking down old connective tissue in the PDL.
21 Therefore, no significant changes in net PDL volumes were observed at the first week, which corresponded to no change in the median percentage of stained PDL (
Figure 2). However, at the molecular level, the mechanosensitive PDL cells can convert mechanical stimuli from the orthodontic preloading force into biological signals, which regulate osteoblastogenesis
22 and osteoclastogenesis.
23 These are consistent with significant increases in the expression of RUNX2 and the RANKL/OPG ratio, respectively, at the first week. The upregulated expression of RUNX2 indicates an induction of mesenchymal cells into differentiated osteoblasts.
5 On the contrary, an increase in the RANKL/OPG ratio, either from the enhanced expression of RANKL due to inflammation within the PDL caused by orthodontic preloading,
8,13 from the decreased expression of OPG in the PDL,
8,13 or both, probably accounts for the significantly increased RANKL/OPG ratio observed at the first week after orthodontic preloading, and would lead to enhanced osteoclastogenesis and subsequent alveolar bone resorption during the initial phase.
24
Subsequently, the lag and post-lag phases involve reorganization of the fibrous attachment apparatus by augmented production of new periodontal fibrils, which takes place throughout the PDL.
20 Consequently, the enhanced synthesis of PDL fibers would be in line with significant increases in the percentage of stained PDL observed at 2 and 4 weeks after orthodontic preloading. In addition, the significant increases in the expressions of RUNX2 and ALP in the PDL observed at 2 and 4 weeks after orthodontic preloading would help recruit and activate new osteoblast progenitors and quiescent bone lining cells to commence the production of new bone matrix.
25 After 4 weeks of orthodontic force application, continuous PDL cell division, resulting in continual collagen synthesis,
26 and high capacities of PDL and alveolar bone remodeling
27 are substantiated by significant and continuing enhancement of PDL volume and the expressions of RUNX2 and ALP as well as the RANKL/OPG ratio.
The kinetic profiles of the induced expressions of RUNX2 and ALP and the increased RANKL/OPG ratio in human PDL tissue upon receiving the leveling force observed in this study are in agreement with the findings of previous in vivo studies that have shown upregulated expression of RUNX2,
28 raised ALP levels,
29 elevated gingival crevicular fluids (GCF) and salivary RANKL levels, and decreased GCF and salivary OPG levels
8,30 upon orthodontic preloading in a time-dependent manner. However, notably, no difference was observed in the expression of RANKL or OPG among the different loading durations (data not shown). This may be attributable to the first observation period at 1 week that may already have been too late to detect any initial changes in the levels of RANKL or OPG, because they have been previously reported to be significantly elevated or lowered, respectively, upon orthodontic loading for 24 hours in the human GCF.
8
This study reveals that orthodontic preloading induces alterations at the macroscopic level, as evidenced by the significant increase in the proliferative PDL tissue, which may help prevent the occurrence of denuded root surfaces due to tooth extraction, possibly leading to a reduction in ankylosis and root resorption after TAT. In addition, orthodontic preloading aids in facilitated tooth extraction,
19 which would decrease the possibility of root fracture of the donor tooth. Moreover, at the molecular level, orthodontic preloading results in significant increases in the expressions of RUNX2 and ALP that are essential for osteoblast differentiation and new bone formation and in the RANKL/OPG ratio that induces bone resorption. All of these molecular changes that affect bone formation and resorption would facilitate adaptation of the donor tooth to the alveolar bone at the recipient site, and promote bone remodeling during the healing of transplanted teeth. Therefore, application of orthodontic preloading results in both PDL proliferation and increased expressions of bone-related molecules. Increased PDL volume ensures easy and atraumatic extraction and provides thick PDL coverage around the radicular portion, which is beneficial for reducing ankylosis risks. Furthermore, increased expressions of bone-related molecules, which are involved in bone remodeling, would modulate the bone healing response. These beneficial effects highlight the clinical implications of orthodontic preloading for good PDL and bone healing, which lead to increased TAT success rate. However, further clinical studies are necessary to investigate the overall benefits of orthodontic preloading on TAT.
Although this study showed no differences in PDL volumes and the expressions of bone biomolecules between 2 and 4 weeks, the optimal orthodontic preloading duration to gain benefits in both PDL volume and bone tissue responses probably is 4 weeks, when all the tested parameters were significantly higher than those in the controls (
Table 2). Additionally, this study is the first to show not only the enhancement of PDL volumes but also the time-course changes in the expressions of important bone biomolecules within human PDL tissues upon orthodontic preloading using a leveling force. Moreover, our investigations on protein expressions were performed in human PDL tissues rather than in the GCF or saliva, as previously reported.
8,30
Table 2
Outcomes of orthodontic preloading compared to those of unloading
|
Outcome |
Loading duration (wk) |
|
|
1 |
2 |
4 |
|
Enhancement of PDL tissue |
NS |
*
|
*
|
|
RUNX2 |
**
|
*
|
*
|
|
ALP |
NS |
*
|
**
|
|
RANKL/OPG ratio |
*
|
NS |
**
|

In this study, a continuous leveling force was used to determine the effect of orthodontic preloading on PDL volumes as well as the expressions of bone biomolecules within the PDL. Despite the leveling force used to generate jiggling movement for the first premolars, the expressions of bone biomolecules likely vary between the pressure and tension sides. This issue was not, however, addressed in this study owing to the collection of total proteins from the PDL tissue of the whole root surface. Consequently, our results should be cautiously interpreted as the total effect of PDL responses upon orthodontic preloading, and not as a specific effect at the pressure or tension side. Other factors, such as the magnitudes and types of force, may differently influence the biological responses of the PDL tissue. Therefore, it would be interesting to further investigate the effect of these factors on the PDL. Moreover, future long-term clinical research is essential to confirm the success rate of TAT after the application of an orthodontic preloading force on the donor tooth for 4 weeks.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download