1. Lim H, Jeong DH, Lee MW, Lee MJ, Shin SW, Yi J. Design of a radiotherapy machine using the 6 MeV C-band standing-wave accelerator. Paper presented at: International Particle Accelerator Conference (IPAC) 2016. 2016 May 10-16; Busan, Korea. p. 1921–1923.
2. Lim H, Jeong DH, Lee MW, Lee MJ, Shin SW, Yi J. Control system of the C-band standing-wave accelerator for the medical application. Paper presented at: International Particle Accelerator Conference (IPAC) 2016. 2016 May 12-16; Busan, Korea. p. 4104–4106.
3. Lim H, Jo W, Lee DE, Lee M, Kim SH, Shin SW, et al. Status of the DIRAMS C-band standing-wave accelerator for a radiotherapy machine. Paper presented at: 9th Asia Forum for Accelerators and Detectors; 2018. 2018 Jan 28-31; Daejeon, Korea. p. 28–31.
4. Kang SK, Kim SH, Kim HC, Lee KH, Lee SJ, Lee DE, et al. Dosimetry system for medical and biological applications of the electron linear accelerator. Paper presented at: The 23rd International Conference on Accelerators and Beam Utilization (ICABU2019). 2019 Nov 13-15; Daejeon, Korea. p. 153.
5. Jang KW, Lee M, Lim H, Kang SK, Lee SJ, Kim SH, et al. 2020; Monte Carlo simulation of an electron irradiation device for medical application of an electron linear accelerator. J Korean Phys Soc. 76:588–591. DOI:
10.3938/jkps.76.588.

6. Schüler E, Trovati S, King G, Lartey F, Rafat M, Villegas M, et al. 2017; Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int J Radiat Oncol Biol Phys. 97:195–203. DOI:
10.1016/j.ijrobp.2016.09.018. PMID:
27816362.

7. International Atomic Energy Agency. 2004. In : IAEA-TECDOC-1386: Emerging applications of radiation processing; 2003 Apr 28-30; Vienna, Austria. International Atomic Energy Agency;Vienna:
8. Petersson K, Jaccard M, Germond JF, Buchillier T, Bochud F, Bourhis J, et al. 2017; High dose-per-pulse electron beam dosimetry - a model to correct for the ion recombination in the Advanced Markus ionization chamber. Med Phys. 44:1157–1167. DOI:
10.1002/mp.12111. PMID:
28094853.

9. Lim H, Lee M, Kim MY, Yi J, Lee M, Kang SK, et al. 2016; Measurement of energy parameters for electron gun heater currents and output dose rate for electron beams from a prototype linac. Prog Med Phys. 27:25–30. DOI:
10.14316/pmp.2016.27.1.25.

10. Lim H, Jeong DH, Lee M, Lee M, Yi J, Yang K, et al. 2016; Solid-state pulse modulator using Marx generator for a medical linac electron-gun. J Instrum. 11:DOI:
10.1088/1748-0221/11/04/P04003.

11. Los Alamos National Laboratory. 2002. The manual of MCNP (Monte Carlo N-particle code system) V2.4.0. Los Alamos National Laboratory;Los Alamos:
12. IAEA TRS-398. 2006. Absorbed dose determination in external beam radiotherapy: an international code of practice for dosimetry based on standards of absorbed dose to water (v.12). International Atomic Energy Agency;Vienna: 75–90.
13. Ding GX, Rogers DWO. 1995. Energy spectra, angular spread and dose distributions of electron beams from various accelerators used in radiotherapy. PIRS-0439. National Research Council of Canada;Ottawa:
14. Patil BJ, Bhoraskar VN, Dhole SD, Chavan ST, Pethe SN, Krishnan R. Optimization of dual scattering foil for 6 to 20 MeV electron beam radiotherapy. Paper presented at: 2011 Particle Accelerator Conference. 2011 March 28-April 1; New York, USA. p. 2157–2159.
15. Khan FM. 2003. The physics of radiation therapy. 3rd ed. In : Wilkins; Lippincott Williams & Wilkins;Philadelphia: p. 315–317.
16. 2016. Standard calibration procedure of gamma irradiation system. Korea Association of Standards & Testing Organizations;Seoul: KASTO 16-80107-102.




 PDF
PDF Citation
Citation Print
Print


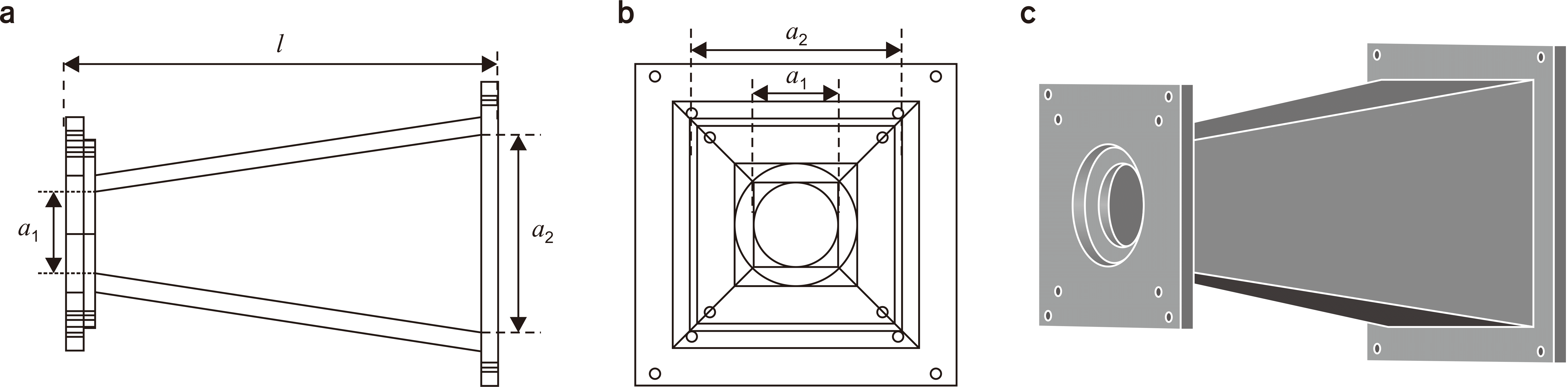
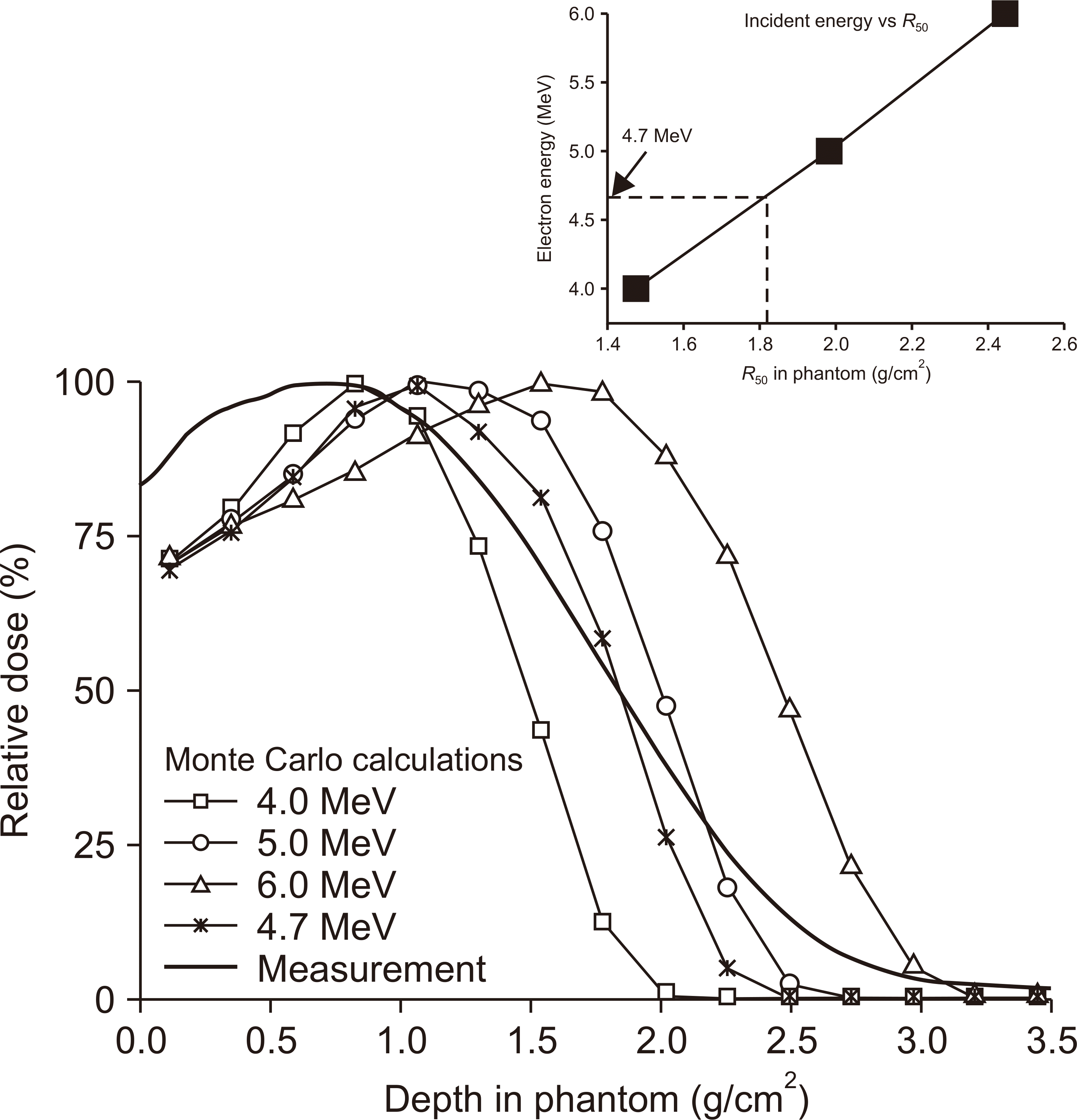
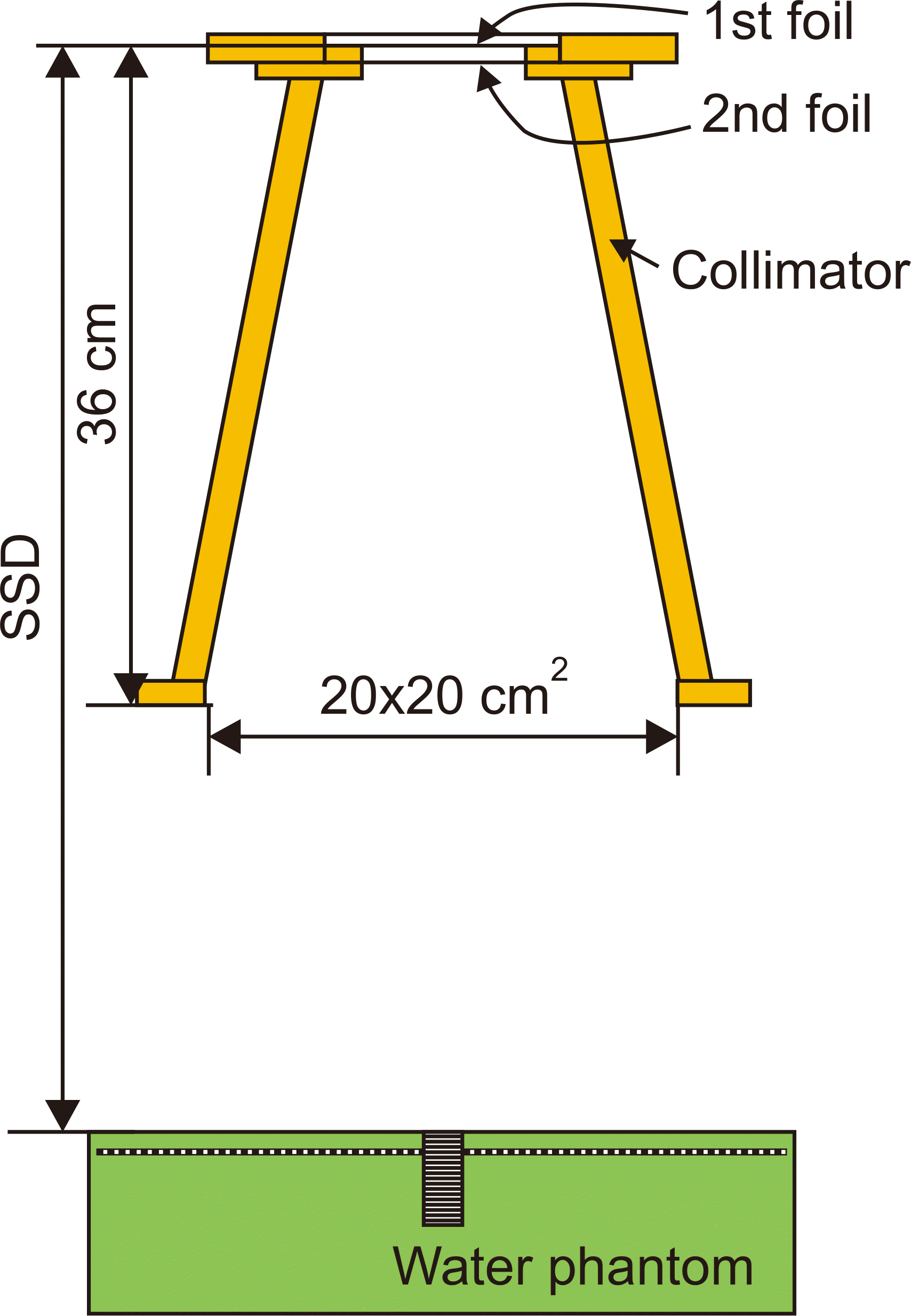
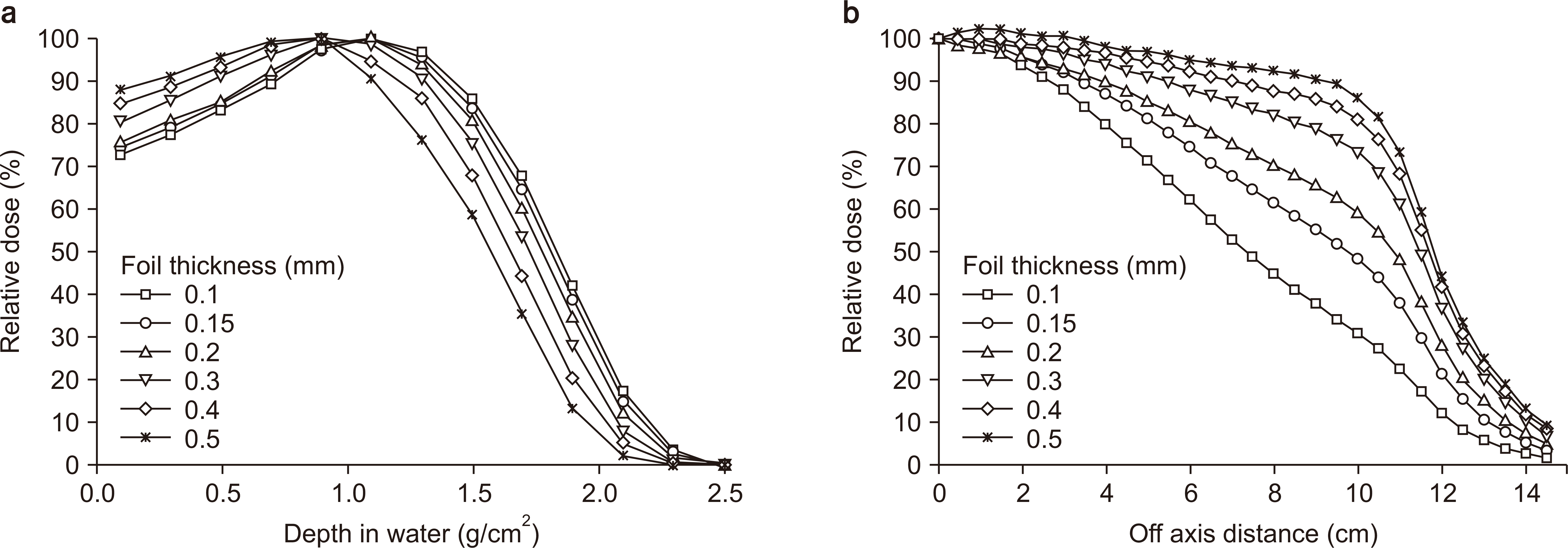

 XML Download
XML Download