1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF diabetes atlas: global estimates for the prevalence of diabetes for
2015 and 2040. Diabetes Res Clin Pract. 2017; 128:40–50. PMID:
28437734.
2. Won JC, Lee JH, Kim JH, Kang ES, Won KC, Kim DJ, Lee MK. Diabetes fact sheet in Korea, 2016: an appraisal of current
status. Diabetes Metab J. 2018; 42:415–424. PMID:
30113146.
3. Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, Cha BY. Prevalence and clinical characteristics of diabetic peripheral neuropathy
in hospital patients with type 2 diabetes in Korea. Diabet Med. 2012; 29:e290–e296. PMID:
22519862.
4. Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, Cha BY. Prevalence and clinical implications of painful diabetic peripheral
neuropathy in type 2 diabetes: results from a nationwide hospital-based study of
diabetic neuropathy in Korea. Diabetes Res Clin Pract. 2014; 103:522–529. PMID:
24438877.
5. Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes
Association. Diabetes Care. 2005; 28:956–962. PMID:
15793206.
6. Ziegler D, Rathmann W, Meisinger C, Dickhaus T, Mielck A. KORA Study Group. Prevalence and risk factors of neuropathic pain in survivors of myocardial
infarction with pre-diabetes and diabetes. The KORA Myocardial Infarction
Registry. Eur J Pain. 2009; 13:582–587. PMID:
18782673.
7. Benatti P, Peluso G, Nicolai R, Calvani M. Polyunsaturated fatty acids: biochemical, nutritional and epigenetic
properties. J Am Coll Nutr. 2004; 23:281–302. PMID:
15310732.
8. Horrobin DF. Essential fatty acids in the management of impaired nerve function in
diabetes. Diabetes. 1997; 46 Suppl 2:S90–S93. PMID:
9285506.
9. Brenner RR. Nutritional and hormonal factors influencing desaturation of essential
fatty acids. Prog Lipid Res. 1981; 20:41–47. PMID:
7342101.
10. Keen H, Payan J, Allawi J, Walker J, Jamal GA, Weir AI, Henderson LM, Bissessar EA, Watkins PJ, Sampson M, Gale EAM, Scarpello J, Boddie HG, Hardy KJ, Thomas PK, Misra P, Halonen JP. The γ-Linolenic Acid Multicenter Trial Group. Treatment of diabetic neuropathy with gamma-linolenic acid. The
gamma-Linolenic Acid Multicenter Trial Group. Diabetes Care. 1993; 16:8–15.
11. Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic
neuropathy. Diabetes. 1997; 46 Suppl 2:S31–S37. PMID:
9285496.
12. Ametov AS, Barinov A, Dyck PJ, Hermann R, Kozlova N, Litchy WJ, Low PA, Nehrdich D, Novosadova M, O'Brien PC, Reljanovic M, Samigullin R, Schuette K, Strokov I, Tritschler HJ, Wessel K, Yakhno N, Ziegler D. SYDNEY Trial Study Group. The sensory symptoms of diabetic polyneuropathy are improved with
alpha-lipoic acid: the SYDNEY trial. Diabetes Care. 2003; 26:770–776. PMID:
12610036.
13. Ziegler D, Sohr CG, Nourooz-Zadeh J. Oxidative stress and antioxidant defense in relation to the severity of
diabetic polyneuropathy and cardiovascular autonomic neuropathy. Diabetes Care. 2004; 27:2178–2183. PMID:
15333481.
14. Bril V, Hirose T, Tomioka S, Buchanan R. Ranirestat Study Group. Ranirestat for the management of diabetic sensorimotor
polyneuropathy. Diabetes Care. 2009; 32:1256–1260. PMID:
19366965.
15. Ropper AH, Gorson KC, Gooch CL, Weinberg DH, Pieczek A, Ware JH, Kershen J, Rogers A, Simovic D, Schratzberger P, Kirchmair R, Losordo D. Vascular endothelial growth factor gene transfer for diabetic
polyneuropathy: a randomized, double-blinded trial. Ann Neurol. 2009; 65:386–393. PMID:
19399887.
16. Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, Bastyr EJ 3rd. MBBQ Study Group. Treatment of symptomatic diabetic peripheral neuropathy with the protein
kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized,
placebo-controlled, double-blind clinical trial. Clin Ther. 2005; 27:1164–1180. PMID:
16199243.
17. Nishikant N, Singh J, Sood S, Vijayshanker V. Effect of gamma linolenic acid pretreatment on diabetic neuropathy in
rats. Int J Basic Clin Pharmacol. 2013; 2:320–325.
18. Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant
alpha-lipoic acid: a meta-analysis. Diabet Med. 2004; 21:114–121. PMID:
14984445.
19. Han T, Bai J, Liu W, Hu Y. A systematic review and meta-analysis of α-lipoic acid in the
treatment of diabetic peripheral neuropathy. Eur J Endocrinol. 2012; 167:465–471. PMID:
22837391.
20. Simon D, Eng PA, Borelli S, Kagi R, Zimmermann C, Zahner C, Drewe J, Hess L, Ferrari G, Lautenschlager S, Wuthrich B, Schmid-Grendelmeier P. Gamma-linolenic acid levels correlate with clinical efficacy of evening
primrose oil in patients with atopic dermatitis. Adv Ther. 2014; 31:180–188. PMID:
24435467.
21. Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, Maus J, Samigullin R. Oral treatment with alpha-lipoic acid improves symptomatic diabetic
polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006; 29:2365–2370. PMID:
17065669.
22. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological
assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994; 17:1281–1289. PMID:
7821168.
23. Park JH, Won JC. Patterns of nerve conduction abnormalities in patients with type 2 diabetes
mellitus according to the clinical phenotype determined by the current perception
threshold. Diabetes Metab J. 2018; 42:519–528. PMID:
30398037.
24. Prendergast JJ, Miranda G, Sanchez M. Improvement of sensory impairment in patients with peripheral
neuropathy. Endocr Pract. 2004; 10:24–30. PMID:
15251618.
25. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria,
estimation of severity, and treatments. Diabetes Care. 2010; 33:2285–2293. PMID:
20876709.
26. Roberts WC. The Friedewald-Levy-Fredrickson formula for calculating low-density
lipoprotein cholesterol, the basis for lipid-lowering therapy. Am J Cardiol. 1988; 62:345–346. PMID:
3165237.
27. Park CY, Kim YS, Oh SJ, Woo JT, Kim SW, Yang IM, Kim JW, Choi YK. Treatment of symptomatic diabetic peripheral neuropathy with thioctic acid
(Thioctacid(R)). Korean Clin Diabetes. 2001; 2:63–74.
28. Schulz KF, Altman DG, Moher D. CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group
randomized trials. Ann Intern Med. 2010; 152:726–732. PMID:
20335313.
29. Staszewski S, Keiser P, Montaner J, Raffi F, Gathe J, Brotas V, Hicks C, Hammer SM, Cooper D, Johnson M, Tortell S, Cutrell A, Thorborn D, Isaacs R, Hetherington S, Steel H, Spreen W. CNAAB3005 International Study Team. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in
antiretroviral-naive HIV-infected adults: a randomized equivalence trial. JAMA. 2001; 285:1155–1163. PMID:
11231744.
30. Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, Samigullin R, Tritschler H, Munzel U, Maus J, Schutte K, Dyck PJ. Efficacy and safety of antioxidant treatment with α-lipoic acid over
4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011; 34:2054–2060. PMID:
21775755.
31. Tesfaye S, Tandan R, Bastyr EJ 3rd, Kles KA, Skljarevski V, Price KL. Ruboxistaurin Study Group. Factors that impact symptomatic diabetic peripheral neuropathy in
placebo-administered patients from two 1-year clinical trials. Diabetes Care. 2007; 30:2626–2632. PMID:
17623822.
32. Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, Khan BV. Irbesartan and lipoic acid improve endothelial function and reduce markers
of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in
Endothelial Dysfunction (ISLAND) study. Circulation. 2005; 111:343–348. PMID:
15655130.
33. Jamal GA, Carmichael H. The effect of gamma-linolenic acid on human diabetic peripheral neuropathy:
a double-blind placebo-controlled trial. Diabet Med. 1990; 7:319–323. PMID:
2159860.
34. Carter JP. Gamma-linolenic acid as a nutrient. Food Technol. 1988; 42:72–82.
35. Omran OM. Histopathological study of evening primrose oil effects on experimental
diabetic neuropathy. Ultrastruct Pathol. 2012; 36:222–227. PMID:
22574767.
36. Nagamatsu M, Nickander KK, Schmelzer JD, Raya A, Wittrock DA, Tritschler H, Low PA. Lipoic acid improves nerve blood flow, reduces oxidative stress, and
improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care. 1995; 18:1160–1167. PMID:
7587852.
37. Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on
endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of
epineurial arterioles of the sciatic nerve. Diabetes. 2001; 50:1927–1937. PMID:
11473057.
38. Hahm JR, Kim BJ, Kim KW. Clinical experience with thioctacid (thioctic acid) in the treatment of
distal symmetric polyneuropathy in Korean diabetic patients. J Diabetes Complications. 2004; 18:79–85. PMID:
15120701.
39. Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. CONSORT Group. Reporting of noninferiority and equivalence randomized trials: an extension
of the CONSORT statement. JAMA. 2006; 295:1152–1160. PMID:
16522836.
40. Veves A, Malik RA. Diabetic neuropathy: clinical management. Dordrecht: Springer Science & Business Media;2008.
41. Boulton AJ. Whither clinical research in diabetic sensorimotor peripheral neuropathy?
Problems of end point selection for clinical trials. Diabetes Care. 2007; 30:2752–2753. PMID:
17901534.
42. Dyck PJ, Norell JE, Tritschler H, Schuette K, Samigullin R, Ziegler D, Bastyr EJ 3rd, Litchy WJ, O'Brien PC. Challenges in design of multicenter trials: end points assessed
longitudinally for change and monotonicity. Diabetes Care. 2007; 30:2619–2625. PMID:
17513707.
43. Dyck PJ, Davies JL, Litchy WJ, O'Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score
in the Rochester Diabetic Neuropathy Study cohort. Neurology. 1997; 49:229–239. PMID:
9222195.
44. Diabetic polyneuropathy in controlled clinical trials: consensus report of
the Peripheral Nerve Society. Ann Neurol. 1995; 38:478–482. PMID:
7668839.
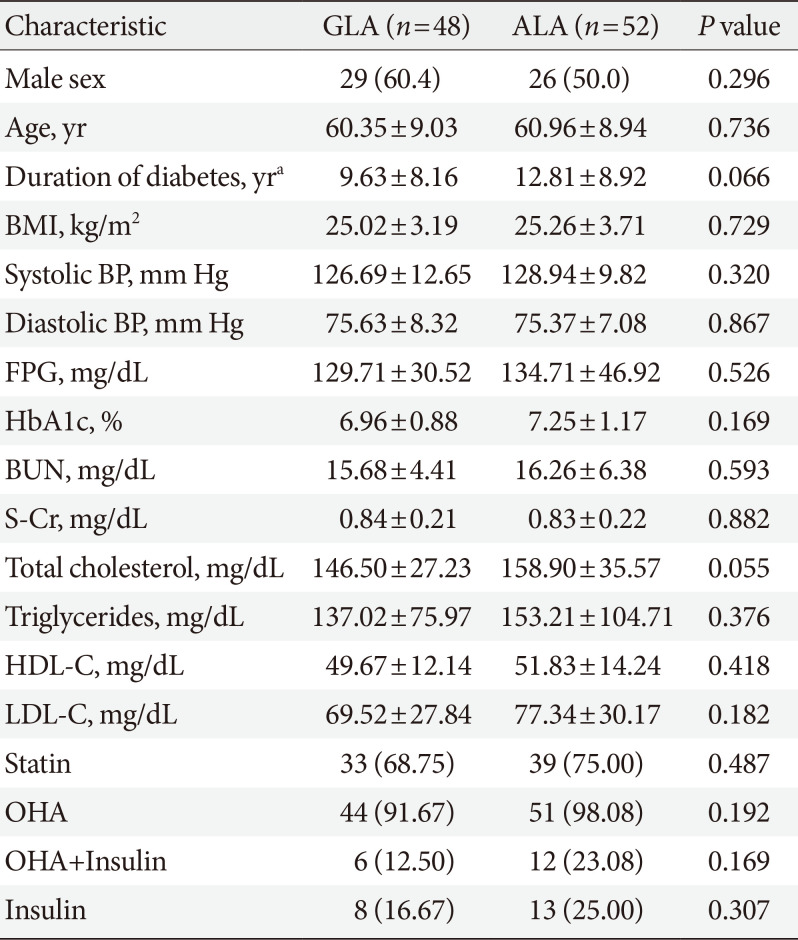
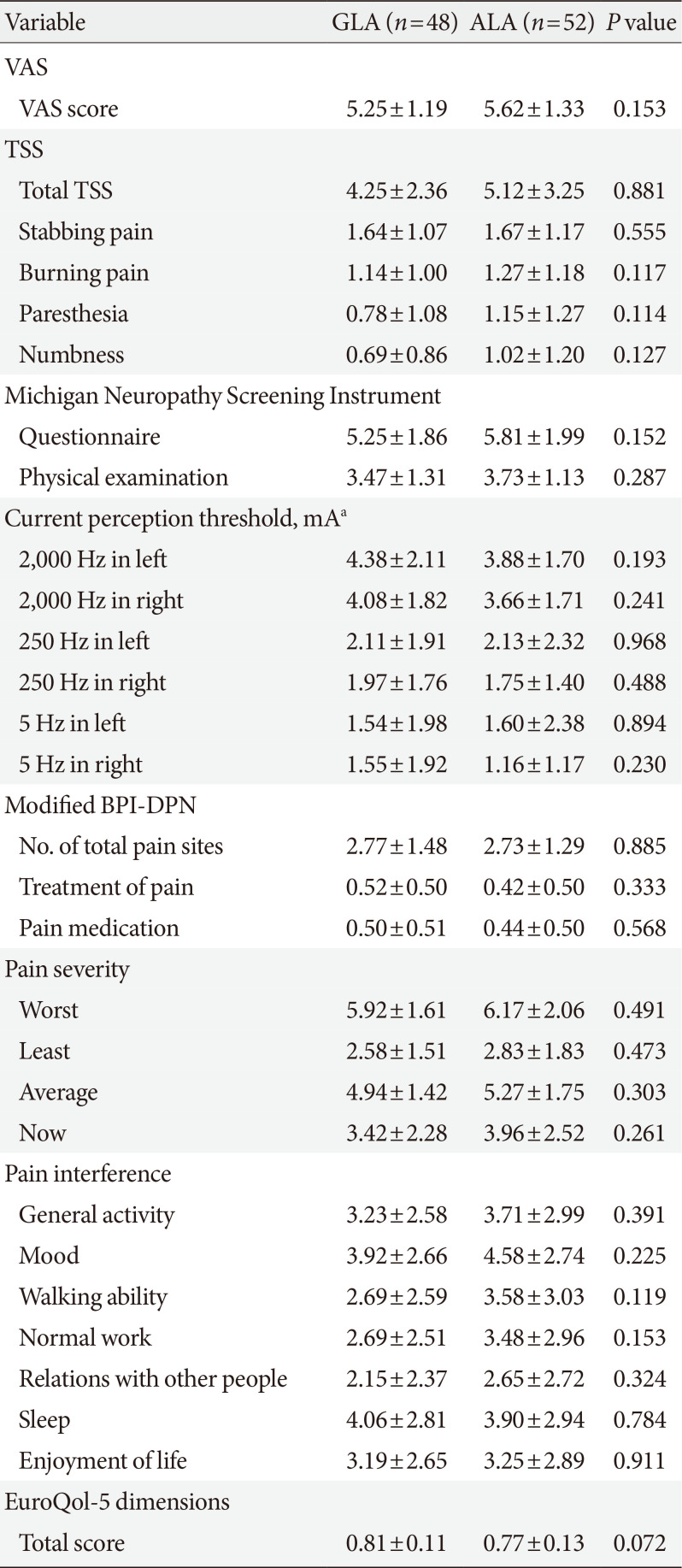
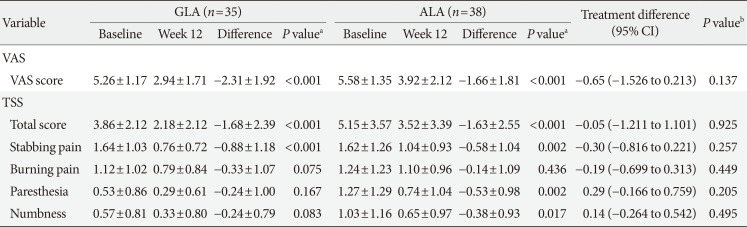
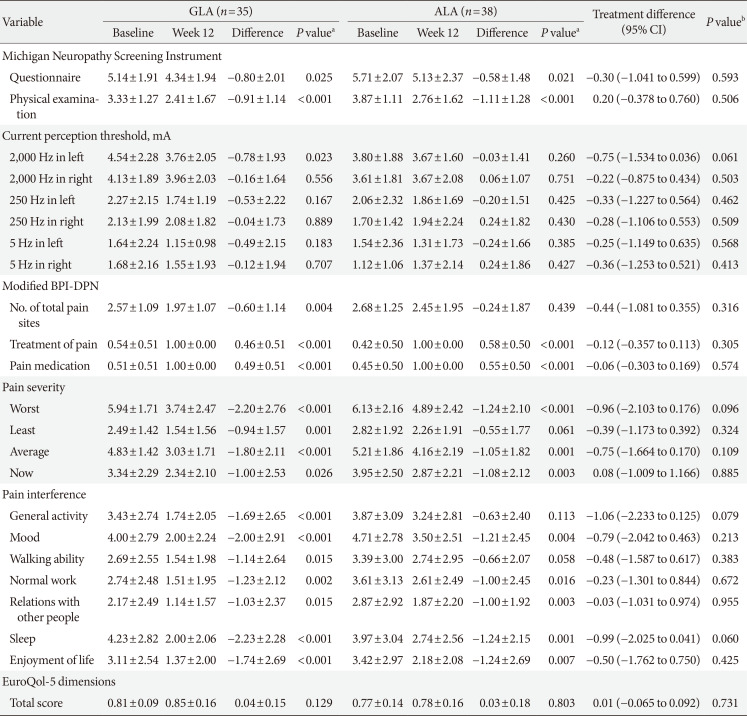




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download