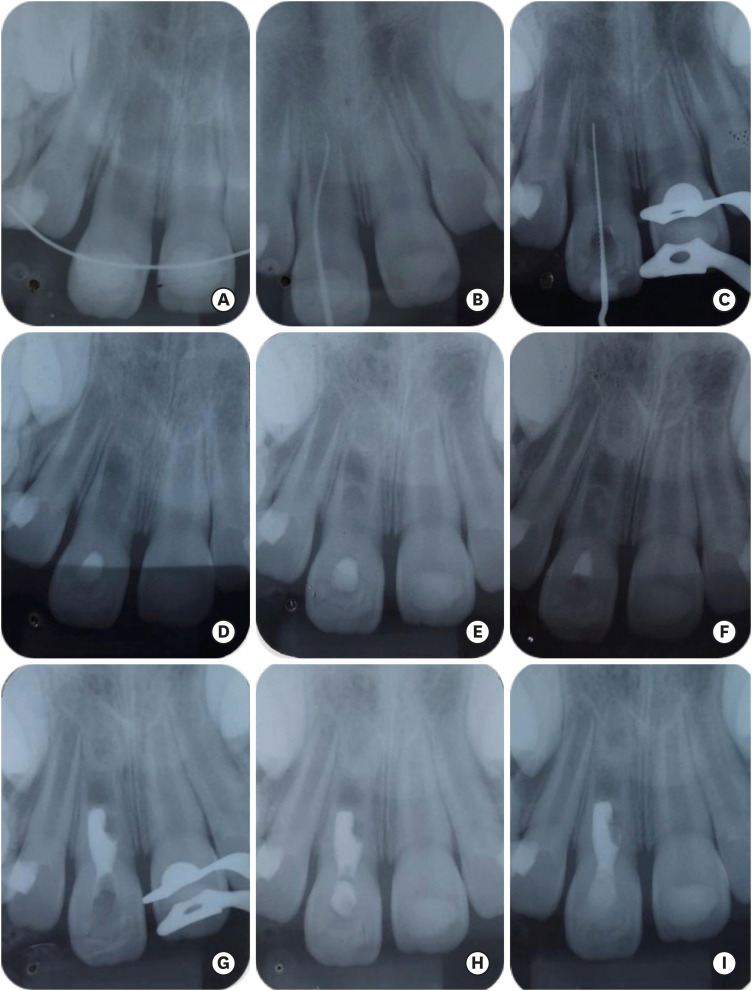
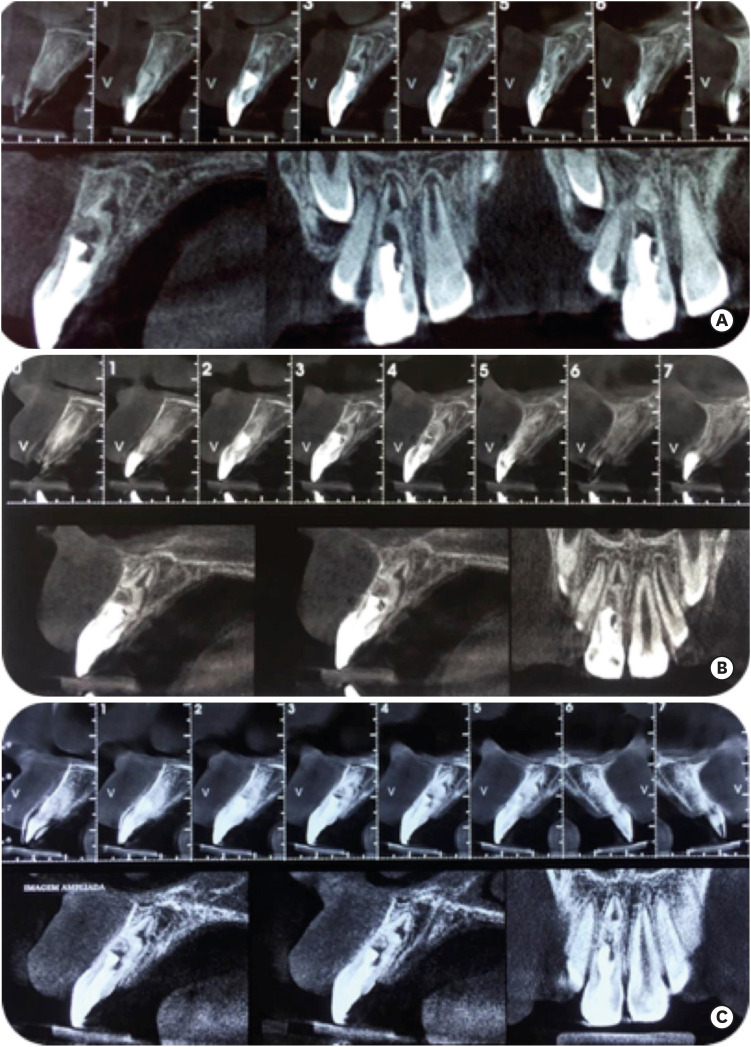
1. Andreasen JO, Andreasen FM, Andersson L. Textbook and color atlas of traumatic injuries to the teeth. 4th ed. Nova Jersey: Wiley-Blackwell;2018.
2. Rodrigues Campos Soares T, de Andrade Risso P, Cople Maia L. Traumatic dental injury in permanent teeth of young patients attended at the federal University of Rio de Janeiro, Brazil. Dent Traumatol. 2014; 30:312–316. PMID:
24289752.

3. Andersson L, Andreasen JO, Day P, Heithersay G, Trope M, DiAngelis AJ, Kenny DJ, Sigurdsson A, Bourguignon C, Flores MT, Hicks ML, Lenzi AR, Malmgren B, Moule AJ, Tsukiboshi M. Guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Pediatr Dent. 2016; 38:369–376. PMID:
27931479.
4. Andersson L, Bodin I. Avulsed human teeth replanted within 15 minutes--a long-term clinical follow-up study. Endod Dent Traumatol. 1990; 6:37–42. PMID:
2390966.
5. Mori GG, Nunes DC, Castilho LR, de Moraes IG, Poi WR. Propolis as storage media for avulsed teeth: microscopic and morphometric analysis in rats. Dent Traumatol. 2010; 26:80–85. PMID:
20089064.

6. Mori GG, Poi WR, Castilho LR. Evaluation of the anti-resorptive ability of an experimental acetazolamide paste for the treatment of late replanted teeth: a study in rats. Dent Traumatol. 2013; 29:34–40. PMID:
22429279.

7. Huang GT. Apexification: the beginning of its end. Int Endod J. 2009; 42:855–866. PMID:
19549154.

8. Diogenes A, Henry MA, Teixeira FB, Hargreaves KM. An update on clinical regenerative endodontics. Endod Topics. 2013; 28:2–23.

9. Namour M, Theys S. Pulp revascularization of immature permanent teeth: a review of the literature and a proposal of a new clinical protocol. Sci World J. 2014; 2014:737503.

10. Moodley T, Patel N. Management of necrotic pulp of immature permanent incisor tooth: a regenerative endodontic treatment protocol: case report. SADJ. 2017; 72:122–125.
11. Thomson A, Kahler B. Regenerative endodontics--biologically-based treatment for immature permanent teeth: a case report and review of the literature. Aust Dent J. 2010; 55:446–452. PMID:
21133946.
12. Nagata JY, Rocha-Lima TF, Gomes BP, Ferraz CC, Zaia AA, Souza-Filho FJ, De Jesus-Soares A. Pulp revascularization for immature replanted teeth: a case report. Aust Dent J. 2015; 60:416–420. PMID:
26219350.

13. Mori GG, Turcio KH, Borro VP, Mariusso AM. Evaluation of the knowledge of tooth avulsion of school professionals from Adamantina, São Paulo, Brazil. Dent Traumatol. 2007; 23:2–5. PMID:
17227372.

14. Silujjai J, Linsuwanont P. Treatment outcomes of apexification or revascularization in nonvital immature permanent teeth: a retrospective study. J Endod. 2017; 43:238–245. PMID:
28132710.

15. Hargreaves KM, Diogenes A, Teixeira FB. Paradigm lost: a perspective on the design and interpretation of regenerative endodontic research. J Endod. 2014; 40(4 Supplement):S65–S69. PMID:
24698697.

16. Zehnder M. Root canal irrigants. J Endod. 2006; 32:389–398. PMID:
16631834.

17. Abuhaimed TS, Abou Neel EA. Sodium hypochlorite irrigation and its effect on bond strength to dentin. BioMed Res Int. 2017; 2017:1930360. PMID:
28904947.

18. Gonçalves LS, Rodrigues RC, Andrade Junior CV, Soares RG, Vettore MV. The effect of sodium hypochlorite and chlorhexidine as irrigant solutions for root canal disinfection: a systematic review of clinical trials. J Endod. 2016; 42:527–532. PMID:
26852149.
19. Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007; 33:377–390. PMID:
17368324.

20. Rôças IN, Provenzano JC, Neves MA, Siqueira JF Jr. Disinfecting effects of rotary instrumentation with either 2.5% sodium hypochlorite or 2% chlorhexidine as the main irrigant: a randomized clinical study. J Endod. 2016; 42:943–947. PMID:
27142579.
21. Kahler B, Chugal N, Lin LM. Alkaline materials and regenerative endodontics: a review. Materials (Basel). 2017; 10:E1389. PMID:
29206139.

22. Iwaya S, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with periradicular abscess after luxation. Dent Traumatol. 2011; 27:55–58. PMID:
21244629.

23. Graham L, Cooper PR, Cassidy N, Nor JE, Sloan AJ, Smith AJ. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials. 2006; 27:2865–2873. PMID:
16427123.

24. Neelakantan P, Romero M, Vera J, Daood U, Khan AU, Yan A, Cheung GSP. Biofilms in endodontics-current status and future directions. Int J Mol Sci. 2017; 18:E1748. PMID:
28800075.

25. Hargreaves KA. Regenerative endodontics. In : Hargreaves KM, Cohen S, editors. Cohen's pathways of the pulp. 10th ed. St. Louis: Elsevier;2011. p. 602–619.
26. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004; 30:196–200. PMID:
15085044.

27. Alsubait S, Al-Haidar S, Al-Sharyan N. A comparison of the discoloration potential for EndoSequence bioceramic root repair material fast set putty and ProRoot MTA in human teeth: an
in vitro study. J Esthet Restor Dent. 2017; 29:59–67. PMID:
27637379.
28. Chen I, Karabucak B, Wang C, Wang HG, Koyama E, Kohli MR, Nah HD, Kim S. Healing after root-end microsurgery by using mineral trioxide aggregate and a new calcium silicate-based bioceramic material as root-end filling materials in dogs. J Endod. 2015; 41:389–399. PMID:
25596728.

29. Shokouhinejad N, Hoseini A, Gorjestani H, Raoof M, Assadian H, Shamshiri AR. Effect of phosphate-buffered saline on push-out bond strength of a new bioceramic sealer to root canal dentin. Dent Res J (Isfahan). 2012; 9:595–599. PMID:
23559925.

30. Ciasca M, Aminoshariae A, Jin G, Montagnese T, Mickel A. A comparison of the cytotoxicity and proinflammatory cytokine production of EndoSequence root repair material and ProRoot mineral trioxide aggregate in human osteoblast cell culture using reverse-transcriptase polymerase chain reaction. J Endod. 2012; 38:486–489. PMID:
22414834.

31. Candeiro GT, Moura-Netto C, D'Almeida-Couto RS, Azambuja-Júnior N, Marques MM, Cai S, Gavini G. Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int Endod J. 2016; 49:858–864. PMID:
26281002.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download