INTRODUCTION
Composites are currently the most commonly preferred direct restoratives, primarily as a result of improvements in adhesive technology in dentistry [
1]. Composites can be used with a minimally invasive technique and provide optical characteristics similar to those of natural teeth; furthermore, composites are easy to handle and insert, facilitating sculpture of the dental anatomy [
2]. Furthermore, layering of the composite is a common clinical technique used during the sculpting and placement of composite restorations [
3]. Despite the broad range of favorable properties that have been anticipated for composites, viscous resin monomers in composites make it difficult to sculpt them to fit the natural anatomical shapes of teeth. Therefore, various modeling agents and equipment have been proposed to improve the adaptation and configuration of composites [
45]. Although this usage is not included in manufacturers' specifications, adhesives have been used as modeling agents for composites.
During incremental layering, adhesion of the composite to the application instrument is a frequently encountered problem [
6]. To solve this problem, studies have shown that resinous monomers used for lubricating instruments or brushes simplify the process of sculpting composites [
67]. For this reason, clinicians have used several lubricants in the incremental layering process to minimize stickiness by wiping the composite instrument with modeling agents, in order to facilitate manipulation and insertion of the composite [
8]. Although these methods are widely used, a potential shortcoming is that these techniques may adversely affect the physical and surface properties of the composite [
257]. However, to the best of our knowledge, no reports in the literature have investigated whether the presence of modeling agents in the composite structure affects the final surface properties of restorations. Moreover, it is unknown whether different compositions of adhesives or modeling agents may affect the surface microhardness, surface roughness, and color stability of composites over time.
The aim of this in vitro study was to investigate the effects of using various modeling agents with composite application instruments on the surface microhardness, surface roughness, and color change of a nano-hybrid composite applied using the layering technique. The null hypotheses tested were that: 1) the use of different modeling agents would not influence the surface microhardness, surface roughness, or color change of a nano-hybrid composite after storage in distilled water or coffee for 1 or 6 weeks, and 2) there would be no difference in any such effects among the modeling agents.
Go to :

MATERIALS AND METHODS
Experimental design and specimen preparation
The surface microhardness, surface roughness, and color change of a nano-hybrid composite were evaluated. Essentia (GC Corp., Tokyo, Japan) nano-hybrid composite (with dark enamel shade) was used to fabricate 64 specimens. The composite was placed using a composite filling spatula in a Teflon mold with dimensions of 12 mm in diameter and 2 mm in thickness for each increment. Subsequently, the top surface of the specimens was treated according to the following application procedures.
• In group 1 (the control group), no modeling agent was applied.
• In group 2, the top surface of the composite specimens was regularized using a humidified sable brush with Modeling Liquid (GC Corp.).
• In Group 3, the top surface of the composite specimens was regularized using a humidified micro-brush applicator with a universal adhesive (G-Premio Bond; GC Corp.).
• In Group 4, the top surface of the composite specimens was regularized using a humidified micro-brush applicator with the primer of a 2-step self-adhesive system/OptiBond XTR (Kerr Corp., Orange, CA, USA).
Table 1 lists the materials, compositions, batch numbers, and their respective manufacturers.
Table 1
Materials used in the study

|
Materials |
Compositions |
Manufacturer |
Lot No. |
|
Essentia (Dark Enamel) (nano-hybrid composite) |
Matrix: UDMA, Bis-EMA, Bis-GMA, TEGDMA |
GC Corp. |
1708182 |
|
Filler: prepolymerized fillers, barium glass, fumed silica (filler content by volume: 65%) |
|
Modeling Liquid (liquid used to model composite materials for direct restorations) |
UDMA, 2-hydroxy-1,3 dimethacryloxy propane, 2-hydroxyethyl methacrylate |
GC Corp. |
1805171 |
|
G-Premio Bond (universal adhesive) |
10-methacryloyloxydecyl dihydrogen phosphate, 4-methacryloxyethyl trimellitate, methacryloyloxyalkyl thiophosphate methylmethacrylate, methacrylate monomer, acetone, water, silica, initiator |
GC Corp. |
1901092 |
|
OptiBond XTR Primer (first step of a 2-step self-adhesive system) |
Glycerol phosphate dimethacrylate, hydrophilic co-monomers, water, ethanol, acetone |
Kerr Corp. |
6350939 |

All specimens were placed between 2 glass slabs and pressed down by the weight of the top slab, with a transparent matrix in between to ensure a smooth surface, and then polymerized for 20 seconds using an LED light-curing unit (Woodpecker Med. Instrument, Guilin, China) with 1,200 mW/cm2 irradiance on the top surface. Then, the specimens were taken out of the Teflon mold, the base of each specimen was numbered with a drill, and the specimens were stored at 37°C in 100% humidity for 24 hours in an incubator. The top surfaces of the specimens were ground with 2,500- and 3,000-grit silicon carbide papers for 20 seconds under tap water. The final thickness of every test specimen was measured with a digital caliper (Digimatic Solar Calipar; Mitutoyo, Takatsu, Japan), and specimens thinner than 1.95 mm or thicker than 2.05 mm were replaced.
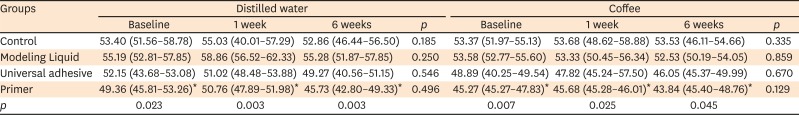
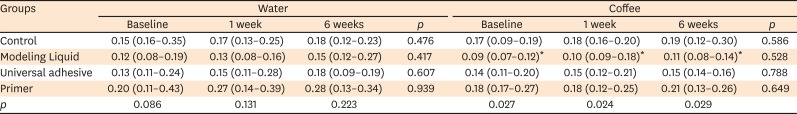
Surface microhardness, surface roughness, and color measurements of the specimens were made (baseline). Then, the specimens were transferred into polyethylene containers containing distilled water or coffee, and the measurements were repeated after 1 and 6 weeks of storage (n = 8).
The staining solution was prepared by adding 7.5 g of coffee (Nescafé Classic; Nestlé, Vevey, Switzerland) to 500 mL of boiling distilled water. All specimens were immersed in a stainless-steel container with coffee solution and stored at 37°C in a dark environment to simulate intraoral conditions [
9]. The staining solution was changed every 2 days throughout the test. After the staining procedure was finished, each specimen was washed under water and dried with an air spray.
Surface microhardness measurements
The microhardness values of the composite surfaces were obtained with a microhardness tester (Shimadzu HMV/2000, Shimadzu Corporation, Kyoto, Japan). The Vickers hardness number (VHN) (kg/mm2) was determined after 24 hours of storage in distilled water and 1 and 6 weeks of storage in distilled water or coffee. Five indentations were made on the top surface under a 200 g load with a 10-second dwell time. The average hardness value for each specimen was then calculated.
Surface roughness measurements
A profilometer (Perthometer M2, Mahr, Göttingen, Germany) was used to determine the surface roughness of the specimens at baseline and after 1 and 6 weeks of storage in distilled water or coffee. The measurement rate was designated as 5.6 mm and the cut-off value as 0.25 mm; then, the measurement needle (10 μm in diameter) was positioned over each test specimen to obtain five readings at different locations of the specimens' surfaces. The mean surface roughness (Ra) value of every specimen was recorded.
Color measurements
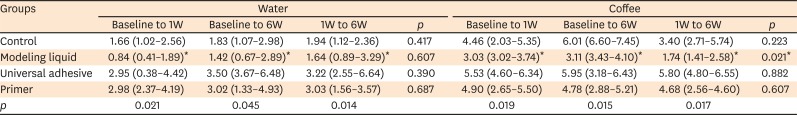
The color distribution (CIE L*, a*, b*, and ΔE*) of each specimen was measured with a spectrophotometer (VITA Easy Shade; Vident, Brea, CA, USA). Measurements were taken at the middle third region of the specimens, and were repeated 3 times at each evaluation. The average value was then calculated. The spectrophotometer was calibrated with a white reflectance standard according to the manufacturer’s protocol before each measurement. The overall color change (ΔE) of the specimens in each group was calculated using the following formula:
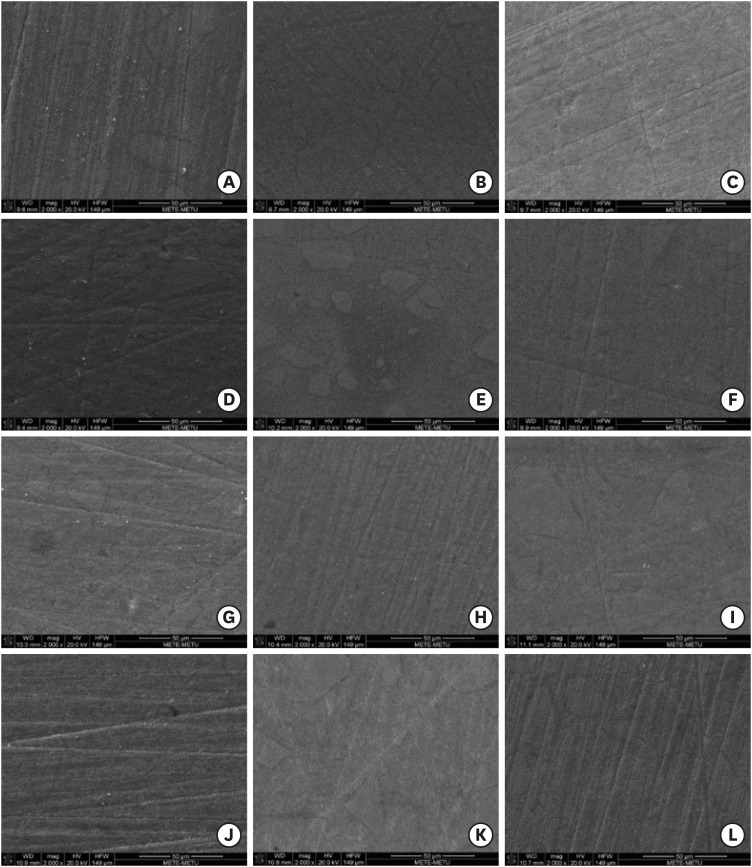
Scanning electron microscopy evaluation
Representative specimens were selected from each group and coated with gold (5 µm) using a sputter coating machine (Quorum Q15OT ES; Quorum Technologies Ltd., East Sussex, UK) for examination with a scanning electron microscope (SEM; Nova NanoSEM 430; FEI Company, Hillsboro, OR, USA) to analyze surface morphology before storage and after 1 and 6 weeks of storage at ×2,000 magnification.
Statistical analysis
Data were analyzed using SPSS version 16 (SPSS Inc., Chicago, IL, USA). The effects of the presence of a modeling agent and storage medium and time on the VHN, Ra, and ΔE of the groups were analyzed using the Kruskal-Wallis non-parametric test, followed by the Bonferroni corrected pairwise comparison test (α = 5%).
Go to :

DISCUSSION
The degree to which modeling agents make it easier to handle composite restorations has led numerous dental professionals to use this technique. Using a brush wetted with a modeling agent makes composites less sticky, resulting in easier and faster application and shaping. Applying the final layer of composites using a wetted brush also enables the clinician to smoothen it efficiently and to achieve a very satisfactory surface. This simplifies the finishing procedure and saves valuable time. However, doubts have arisen regarding possible changes in the characteristics and properties of composites caused by the application of these agents, particularly changes related to the surface microhardness, roughness, and color stability of restorations over time [
410]. Thus, the present study evaluated the effects of different kinds of modeling agents on the surface microhardness, surface roughness, and color change of a nano-hybrid composite exposed to coffee staining over time. Favorable results were found in comparison to the conventional technique (without the use of modeling), with similar or superior surface physical properties and color variations.
In the analysis according to the presence of a modeling agent, the use of various modeling agents to prepare specimens influenced the surface microhardness, surface roughness and overall color change of the tested nano-hybrid composite, in comparison with the specimens prepared without a modeling agent; therefore, the first null hypothesis of the study was rejected.
The use of low-viscosity materials (
e.g., resin adhesives) as modeling agents for resin composites has been reported to provide a way of achieving good clinical results with adequate insertion, and these materials have been reported to be especially useful for modeling of composite increments, but scientific evidence supporting their use for this purpose is lacking. Considering that adhesives have been mainly used as modeling agents for composites during the build-up process of anterior esthetic restorations, it would be interesting to determine whether this technique may negatively affect the surface and color stability properties of esthetic nano-hybrid composites. The present research is a pioneer study showing the positive effects of using the tested Modeling Liquid to sculpt and to model the nano-hybrid composite [
11].
In the present study, the surface microhardness of the nano-hybrid composite was similar, regardless of whether the universal adhesive, Modeling Liquid, or no modeling agent was used. In contrast, using the primer of the self-adhesive system influenced the surface microhardness negatively (
Table 2). One explanation for this difference in surface microhardness of the nano-hybrid composite is related to the pH value (1.6) of this alternative modeling agent. Although the finishing and polishing processes remove the resin-rich layer of a composite specimen cured under a polyester matrix strip and might remove the top surface of the composite, which was affected by the modeling agent, a softening effect of the primer due to its low pH was seen. It is suggested that the application of universal adhesive or Modeling Liquid within the composite surface avoided the occurrence of defects or voids during the modeling of the material, making the composite more resistant to degradation [
12]. Although no clear structural and morphological differences could be detected among specimens prepared with or without a modeling agent, specimens prepared with an adhesive primer exhibited considerably lower mechanical stability than the other groups. However, the findings of this study regarding surface microhardness are in disagreement with those of the study performed by Tuncer
et al. [
10], who reported that significantly lower microhardness values were achieved in composite specimens that were prepared with modeling resin under a polyester matrix strip. They explained their findings on the basis of a resin-rich layer on the surfaces of the tested composites caused by the modeling resin.
Some liquid resins have been suggested as a way to achieve smoother surfaces in composites [
1314]. However, it has proven difficult to obtain a regular surface with liquid resins [
9]. In the present study, while the Ra values of the control, Modeling Liquid, and universal adhesive test groups were below the plaque accumulation threshold level of 0.20 mm [
15], the groups where Modeling Liquid and universal adhesive were used exhibited similar Ra values to those of the control group. The present study also showed statistically significantly higher Ra values for the self-adhesive system group than for the other groups. The variations in the Ra values of the tested modeling agents in the present study may also be attributed to the effects of their low pH. The low pH value of modeling agents can cause deterioration of the top surface of the composite, potentially leading to the formation of microcracks and removal of the resin matrix.
These findings confirm the superiority of the tested universal adhesive or Modeling Liquid as a modeling agent for improving the surface texture of nano-hybrid composites and avoiding irregularities and defects on the top surface of composite restorations. This can be attributed to enhanced adaptation of the composite layers, which hinders the composite from sticking to the hand tools.
The results of this study also showed that the storage medium (coffee) did not affect the surface roughness of the nano-hybrid composite at the investigated storage times. One limitation of this study is that the maximum storage time was 6 weeks, as long-term aging studies have shown that deterioration of nano-hybrid composite surfaces may occur after long periods of storage [
1617]. However, the Ra values in the Modeling Liquid group were influenced less than those in the other groups by storage in coffee. This finding could be explained by the similar composition of Modeling Liquid and the nano-hybrid composite resin matrix.
A minimum ΔE* is one of the most important characteristics desired for composite restorations, especially those involving the anterior teeth. However, composites commonly suffer from degradation because of their polymeric structure, which results in a compromised color appearance over time. According to recent studies, the use of modeling agents (
e.g., adhesive resins) between composite layers may be a useful strategy to reduce or delay composite staining [
511]. Thus, one of the purposes of the present study was to investigate whether the color shade of a nano-hybrid composite prepared with a modeling agent would change after 6 weeks of storage in distilled water or coffee. In the overall analysis, the ΔE* of the nano-hybrid composite depended on the type of modeling agent used. Only ΔE* values in the Modeling Liquid group remained stable over time regardless of storage medium; by contrast, the control, universal adhesive and primer of the self-adhesive system groups showed increased ΔE* values after 6 weeks of storage in water and coffee compared with the baseline values. These findings demonstrate that the type of modeling agent used for layering plays an important role in the color stability of the composite.
Similarly to specimens prepared with a universal adhesive, the specimens from the primer of the self-adhesive group and the control specimens demonstrated increased ΔE* values after 6 weeks of water storage, which may be explained by the strong tendency of polymer-based materials to undergo hydrolysis [
18]. Water is a potent solvent capable of degrading the intermolecular bonds of composites [
19]. The use of a hydrophobic unfilled resin (Modeling Liquid) as a modeling agent also helped keep the color of the composite stable over time, even after 6 weeks of water or coffee storage, likely because the modeling liquid exerted a protective effect against hydrolysis. This finding is corroborated by recent studies [
411].
In the present study, the primer of a 2-step self-adhesive system that was tested as a modeling agent for sculpting composite specimens contained a hydrophilic co-monomer, water, and ethanol. The presence of water in this material possibly contributed to its attraction of additional water into the resin matrix, which softened the top surface of the specimens where the material was applied with a micro-brush. For this reason, the microhardness of this group was significantly lower than that of the other groups. Previous studies have demonstrated that as the volume of unfilled resin increases, a greater amount of water is absorbed into the matrix [
2021]. Thus, it is possible that the increased hydrophilic co-monomer content of the modeling agent was the mechanism responsible for the decreased microhardness and increased color change. Additionally, similar to our study, previous studies have mentioned that the hydrophilic characteristics of these monomers and solvents made restorations more prone to absorbing staining pigments and to changing color over time [
7101122].
The pH values of the universal adhesive and the primer of the 2-step self-adhesive system tested in this study were 2.1 and 1.6, respectively. However, the pH value of the universal adhesive did not affect the tested parameters to the same extent as the primer of the 2-step self-adhesive system. This could be explained by the nano-filler content of the universal adhesive.
The optical appearance of composite restorations should ideally not change over time. In a study by Tuncer
et al. [
10], the color stability of composites was negatively affected by covering them with a superficial layer of a modeling agent. The optical properties of composites are not expected to remain stable over time, especially considering the degradation that composites may undergo after placement in the oral environment [
23]. Color shade is the main property susceptible to modification, and special attention must be given to restorations prepared using modeling agents because these agents may influence the color of composites after aging [
4].
Although the specimens prepared using Modeling Liquid showed better surface and color stability properties than the other groups, an analysis of SEM images (
Figure 1) demonstrated that, after storage in distilled water or coffee, the specimens prepared with Modeling Liquid did not exhibit visibly smoother surfaces than the other groups.
Important limitations of this study include the fact that only 1 type of composite was tested, the use of only a 6-week storage time, and not applying thermocycling for aging.
Taking the findings together, the first null hypothesis tested here was rejected. Although the composites prepared with the primer of the self-adhesive system showed reduced surface microhardness after aging in both storage media and at both storage times, those prepared with Modeling Liquid exhibited a more planar surface in coffee and greater color stability in both storage media and at both storage times. The second null hypothesis tested here was accepted, because storage in distilled water or coffee for 1 and 6 weeks did not affect the surface microhardness, surface roughness, or color change of a nano-hybrid composite.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download