1. Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. 2002; Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 302:381–389. DOI:
10.1124/jpet.102.033175. PMID:
12065741.

2. Urban JD, Vargas GA, von Zastrow M, Mailman RB. 2007; Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 32:67–77. DOI:
10.1038/sj.npp.1301071. PMID:
16554739.

3. Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. 2003; Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 28:1400–1411. DOI:
10.1038/sj.npp.1300203. PMID:
12784105.

4. Kirschbaum KM, Müller MJ, Malevani J, Mobascher A, Burchardt C, Piel M, Hiemke C. 2008; Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J Biol Psychiatry. 9:212–218. DOI:
10.1080/15622970701361255. PMID:
17853280.

5. Stahl SM. 2001; Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 2: illustrating their mechanism of action. J Clin Psychiatry. 62:923–924. DOI:
10.4088/JCP.v62n1201. PMID:
11780870.

7. Torgovnick J, Sethi NK, Arsura E. 2008; Aripiprazole-induced orthostatic hypotension and cardiac arrhythmia. Psychiatry Clin Neurosci. 62:485. DOI:
10.1111/j.1440-1819.2008.01833.x. PMID:
18778452.
8. Shao Q, Quan W, Jia X, Chen J, Ma S, Zhang X. 2015; Severe arrhythmia induced by orally disintegrating aripiprazole tablets (BosiqingⓇ): a case report. Neuropsychiatr Dis Treat. 11:3019–3021. DOI:
10.2147/NDT.S91771. PMID:
26677328.
9. Nelson S, Leung JG. 2013; Torsades de pointes after administration of low-dose aripiprazole. Ann Pharmacother. 47:e11. DOI:
10.1345/aph.1R387. PMID:
23362038.

11. Huang XP, Mangano T, Hufeisen S, Setola V, Roth BL. 2010; Identification of human Ether-à-go-go related gene modulators by three screening platforms in an academic drug-discovery setting. Assay Drug Dev Technol. 8:727–742. DOI:
10.1089/adt.2010.0331. PMID:
21158687.
12. Serôdio P, Vega-Saenz de Miera E, Rudy B. 1996; Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. J Neurophysiol. 75:2174–2179. DOI:
10.1152/jn.1996.75.5.2174. PMID:
8734615.
13. Ohya S, Tanaka M, Oku T, Asai Y, Watanabe M, Giles WR, Imaizumi Y. 1997; Molecular cloning and tissue distribution of an alternatively spliced variant of an A-type K+ channel alpha-subunit, Kv4.3 in the rat. FEBS Lett. 420:47–53. DOI:
10.1016/S0014-5793(97)01483-X. PMID:
9450548.
14. O'Donovan B, Adeluyi A, Anderson EL, Cole RD, Turner JR, Ortinski PI. 2019; Altered gating of Kv1.4 in the nucleus accumbens suppresses motivation for reward. Elife. 8:e47870. DOI:
10.7554/eLife.47870. PMID:
31487241. PMCID:
PMC6728144.
15. Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. 2001; Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A. 98:13373–13378. DOI:
10.1073/pnas.231376298. PMID:
11698689. PMCID:
PMC60878.

16. Guo W, Li H, London B, Nerbonne JM. 2000; Functional consequences of elimination of i(to,f) and i(to,s): early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 alpha subunit. Circ Res. 87:73–79. DOI:
10.1161/01.RES.87.1.73. PMID:
10884375.
17. Ahn HS, Kim SE, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Hahn SJ. 2006; Interaction of riluzole with the closed inactivated state of Kv4.3 channels. J Pharmacol Exp Ther. 319:323–331. DOI:
10.1124/jpet.106.106724. PMID:
16815868.

18. Chae YJ, Kim DH, Lee HJ, Sung KW, Kwon OJ, Hahn SJ. 2015; Raloxifene inhibits cloned Kv4.3 channels in an estrogen receptor-independent manner. Pflugers Arch. 467:1663–1676. DOI:
10.1007/s00424-014-1602-3. PMID:
25231973.

20. Choi BH, Choi JS, Ahn HS, Kim MJ, Rhie DJ, Yoon SH, Min DS, Jo YH, Kim MS, Hahn SJ. 2003; Fluoxetine blocks cloned neuronal A-type K+ channels Kv1.4. Neuroreport. 14:2451–2455. DOI:
10.1097/00001756-200312190-00032. PMID:
14663209.

22. Castle NA. 1990; Bupivacaine inhibits the transient outward K+ current but not the inward rectifier in rat ventricular myocytes. J Pharmacol Exp Ther. 255:1038–1046. PMID:
2262891.
23. Wang Z, Fermini B, Nattel S. 1995; Effects of flecainide, quinidine, and 4-aminopyridine on transient outward and ultrarapid delayed rectifier currents in human atrial myocytes. J Pharmacol Exp Ther. 272:184–196. PMID:
7815332.
24. Wood MD, Scott C, Clarke K, Westaway J, Davies CH, Reavill C, Hill M, Rourke C, Newson M, Jones DN, Forbes IT, Gribble A. 2006; Aripiprazole and its human metabolite are partial agonists at the human dopamine D2 receptor, but the rodent metabolite displays antagonist properties. Eur J Pharmacol. 546:88–94. DOI:
10.1016/j.ejphar.2006.07.008. PMID:
16925992.

25. Kim SE, Ahn HS, Choi BH, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Hahn SJ. 2007; Open channel block of A-type, kv4.3, and delayed rectifier K+ channels, Kv1.3 and Kv3.1, by sibutramine. J Pharmacol Exp Ther. 321:753–762. DOI:
10.1124/jpet.106.117747. PMID:
17312186.

26. Petersen KR, Nerbonne JM. 1999; Expression environment determines K+ current properties: Kv1 and Kv4 alpha-subunit-induced K+ currents in mammalian cell lines and cardiac myocytes. Pflugers Arch. 437:381–392. DOI:
10.1007/s004240050792. PMID:
9914394.
27. Roeper J, Lorra C, Pongs O. 1997; Frequency-dependent inactivation of mammalian A-type K+ channel KV1.4 regulated by Ca
2+/calmodulin-dependent protein kinase. J Neurosci. 17:3379–3391. DOI:
10.1523/JNEUROSCI.17-10-03379.1997. PMID:
9133364. PMCID:
PMC6573714.
28. Wissmann R, Bildl W, Oliver D, Beyermann M, Kalbitzer HR, Bentrop D, Fakler B. 2003; Solution structure and function of the "tandem inactivation domain" of the neuronal A-type potassium channel Kv1.4. J Biol Chem. 278:16142–16150. DOI:
10.1074/jbc.M210191200. PMID:
12590144.

29. Ishii K, Nakamura Y, Taira N. Kondoh Si. 1997; A mammalian transient type K+ channel, rat Kv1.4, has two potential domains that could produce rapid inactivation. J Biol Chem. 272:19333–19338. DOI:
10.1074/jbc.272.31.19333. PMID:
9235930.
30. Ruppersberg JP, Frank R, Pongs O, Stocker M. 1991; Cloned neuronal IK(A) channels reopen during recovery from inactivation. Nature. 353:657–660. DOI:
10.1038/353657a0. PMID:
1922383.

31. Lopez GA, Jan YN, Jan LY. 1994; Evidence that the S6 segment of the Shaker voltage-gated K+ channel comprises part of the pore. Nature. 367:179–182. DOI:
10.1038/367179a0. PMID:
8114915.

32. Bett GC, Rasmusson RL. 2004; Inactivation and recovery in Kv1.4 K+ channels: lipophilic interactions at the intracellular mouth of the pore. J Physiol. 556(Pt 1):109–120. DOI:
10.1113/jphysiol.2003.055012. PMID:
14608006. PMCID:
PMC1664896.
33. Madhav NVS, Ojha A, Jaiswal V. 2018; A smart approach for delivery of aripiprazole via oro-soft palatal mucosal route for improved therapeutic efficacy. Braz J Pharm Sci. 54:e17382. DOI:
10.1590/s2175-97902018000317382.

35. Tytgat J, Maertens C, Daenens P. 1997; Effect of fluoxetine on a neuronal, voltage-dependent potassium channel (Kv1.1). Br J Pharmacol. 122:1417–1424. DOI:
10.1038/sj.bjp.0701545. PMID:
9421290. PMCID:
PMC1565099.

36. Choi JS, Hahn SJ, Rhie DJ, Yoon SH, Jo YH, Kim MS. 1999; Mechanism of fluoxetine block of cloned voltage-activated potassium channel Kv1.3. J Pharmacol Exp Ther. 291:1–6. PMID:
10490879.
37. Sparshatt A, Taylor D, Patel MX, Kapur S. 2010; A systematic review of aripiprazole--dose, plasma concentration, receptor occupancy, and response: implications for therapeutic drug monitoring. J Clin Psychiatry. 71:1447–1456. DOI:
10.4088/JCP.09r05060gre. PMID:
20584524.
38. Citrome L, Josiassen R, Bark N, Salazar DE, Mallikaarjun S. 2005; Pharmacokinetics of aripiprazole and concomitant lithium and valproate. J Clin Pharmacol. 45:89–93. DOI:
10.1177/0091270004269870. PMID:
15601809.

39. Boulton DW, Kollia G, Mallikaarjun S, Komoroski B, Sharma A, Kovalick LJ, Reeves RA. 2008; Pharmacokinetics and tolerability of intramuscular, oral and intravenous aripiprazole in healthy subjects and in patients with schizophrenia. Clin Pharmacokinet. 47:475–485. DOI:
10.2165/00003088-200847070-00004. PMID:
18563956.
40. Sheng M, Tsaur ML, Jan YN, Jan LY. 1992; Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 9:271–284. DOI:
10.1016/0896-6273(92)90166-B. PMID:
1497894.

41. Zemel BM, Ritter DM, Covarrubias M, Muqeem T. 2018; A-Type KV channels in dorsal root ganglion neurons: diversity, function, and dysfunction. Front Mol Neurosci. 11:253. DOI:
10.3389/fnmol.2018.00253. PMID:
30127716. PMCID:
PMC6088260.

42. Norris AJ, Nerbonne JM. 2010; Molecular dissection of I(A) in cortical pyramidal neurons reveals three distinct components encoded by Kv4.2, Kv4.3, and Kv1.4 alpha-subunits. J Neurosci. 30:5092–5101. DOI:
10.1523/JNEUROSCI.5890-09.2010. PMID:
20371829. PMCID:
PMC2862390.
43. Burkhalter A, Gonchar Y, Mellor RL, Nerbonne JM. 2006; Differential expression of
IA channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses. J Neurosci. 26:12274–12282. DOI:
10.1523/JNEUROSCI.2599-06.2006. PMID:
17122053.
44. Hoffman DA, Magee JC, Colbert CM, Johnston D. 1997; K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 387:869–875. DOI:
10.1038/43119. PMID:
9202119.

45. Patel SP, Campbell DL. 2005; Transient outward potassium current, 'Ito', phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. J Physiol. 569(Pt 1):7–39. DOI:
10.1113/jphysiol.2005.086223. PMID:
15831535. PMCID:
PMC1464208.

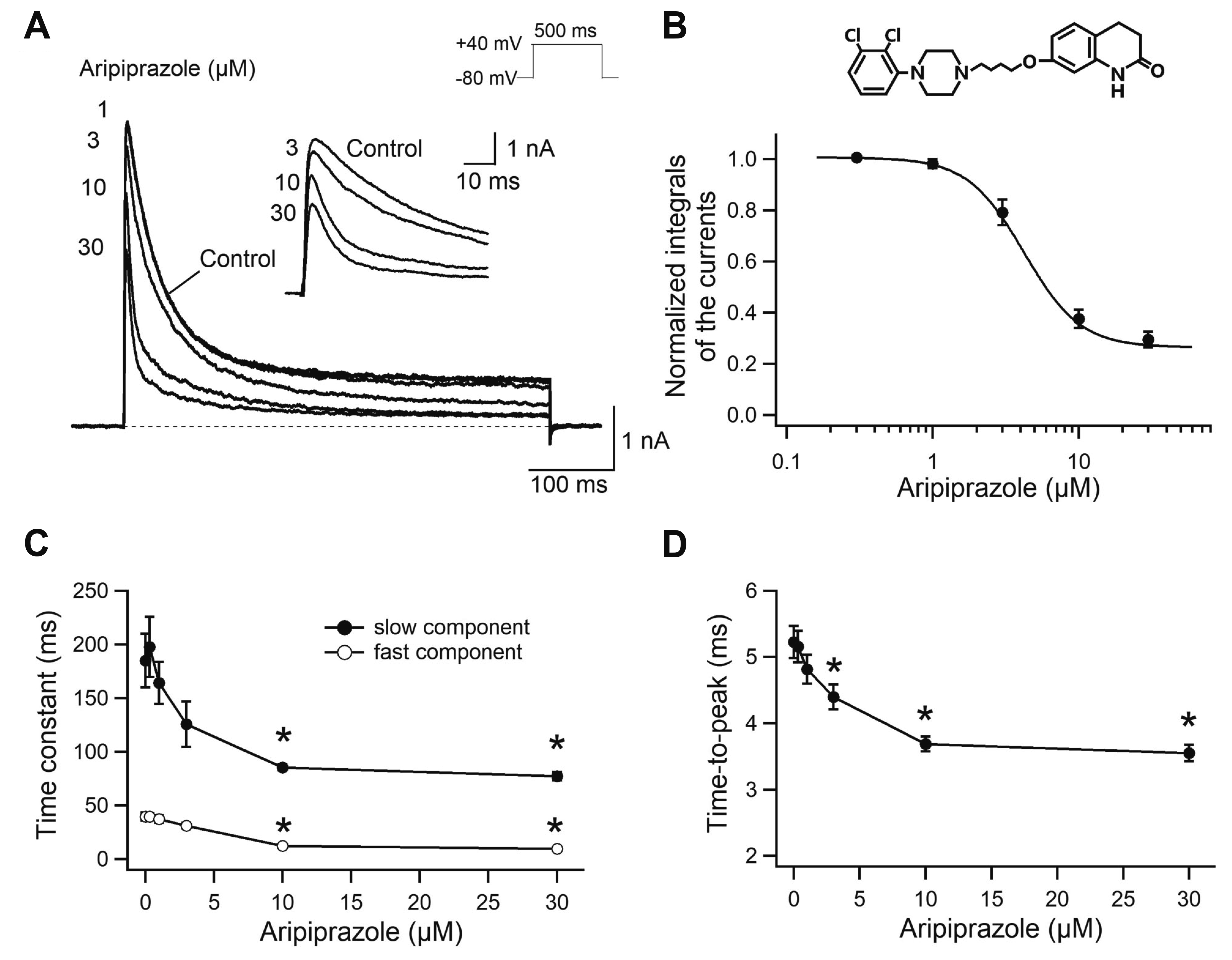
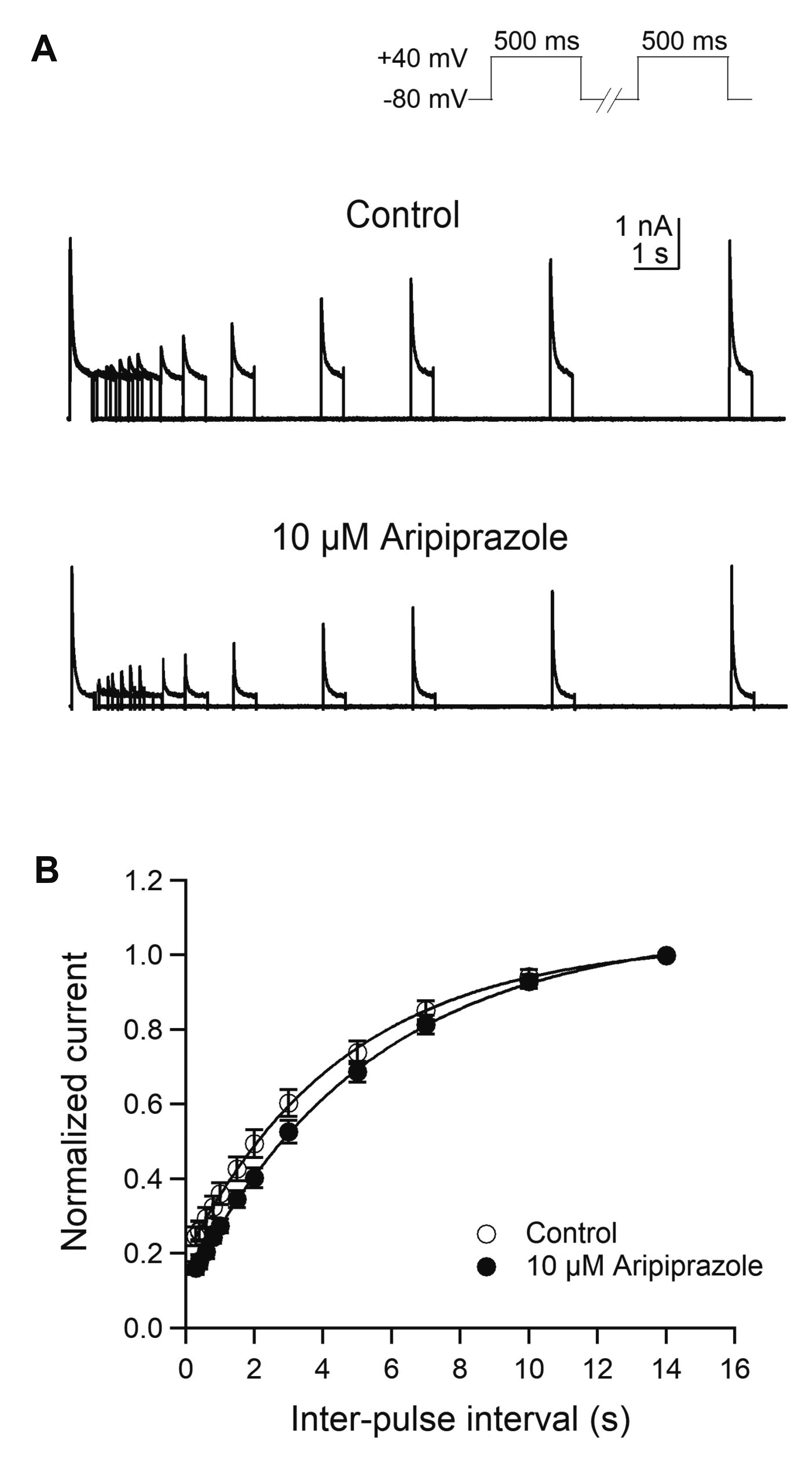
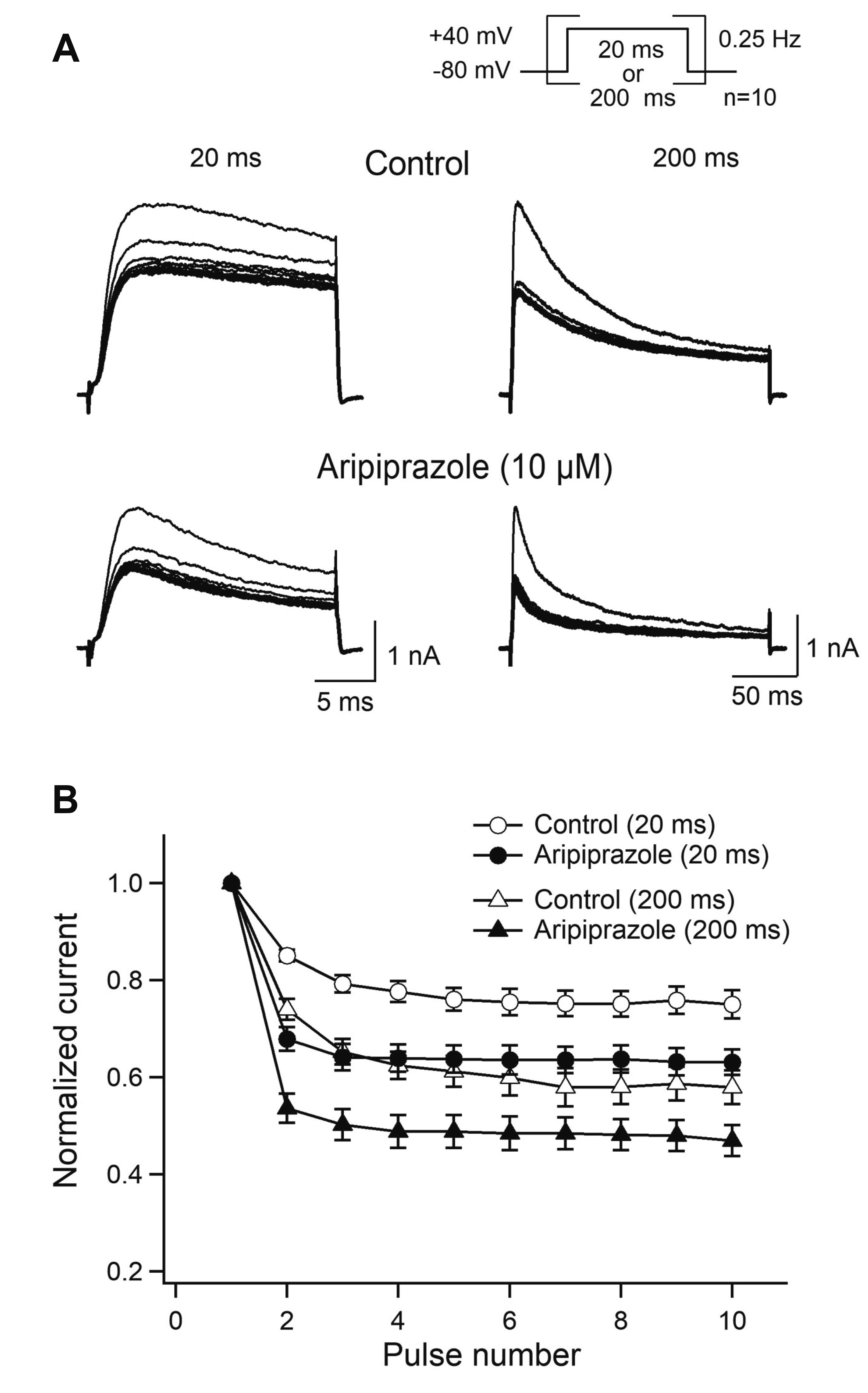




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download