INTRODUCTION
METHODS
Slice preparation
Whole-cell patch-clamp recording
FM1-43 unloading
Drugs
Statistical analysis
RESULTS
Layer-specific activation of layer 2/3 pyramidal neurons with local electrical stimulation
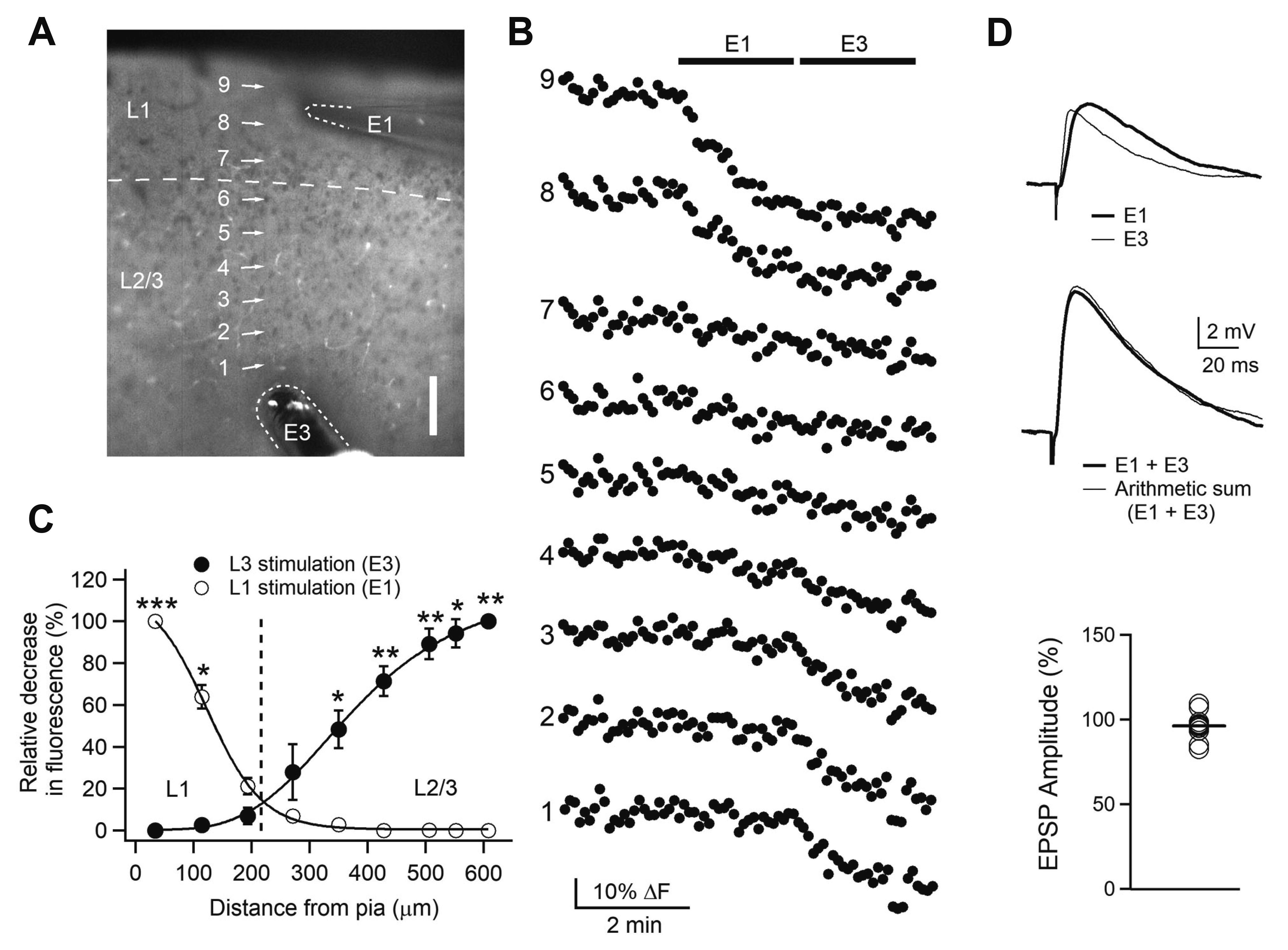 | Fig. 1Layer-specific activation of Layer 2/3 pyramidal neurons with local electrical stimulation.Experimental configuration (10× objective image) showing numbered regions of interest (ROIs) with stimulating electrodes marked with E1 for layer 1 (L1) stimulation and E3 for layer 3 (L3) stimulation. Scale = 100 μm. (B) Fluorescence changes in ROIs with time (5-sec interval). E1 and E3 were applied at 2 Hz (bars). Data are from a representative experiment (n = 3). (C) Spatial profiles of relative fluorescence changes against distance from the pia. The relative decrease in fluorescence to the maximal decrease was plotted. *p < 0.05, **p < 0.01, ***p < 0.001 compared with L1 or L3 stimulation. (D) Activation of non-overlapping synapses. Upper traces show representative EPSPs evoked by E1 and E3. Middle traces show representative EPSP evoked by combining E1 with E3 and arithmetic sum of corresponding E1-EPSP and E3-EPSP. Lower panel shows individual data of the amplitude of arithmetic sum relative to the amplitude of EPSPs evoked by combined E1 and E3 (n = 12). EPSP, excitatory postsynaptic potential.
|
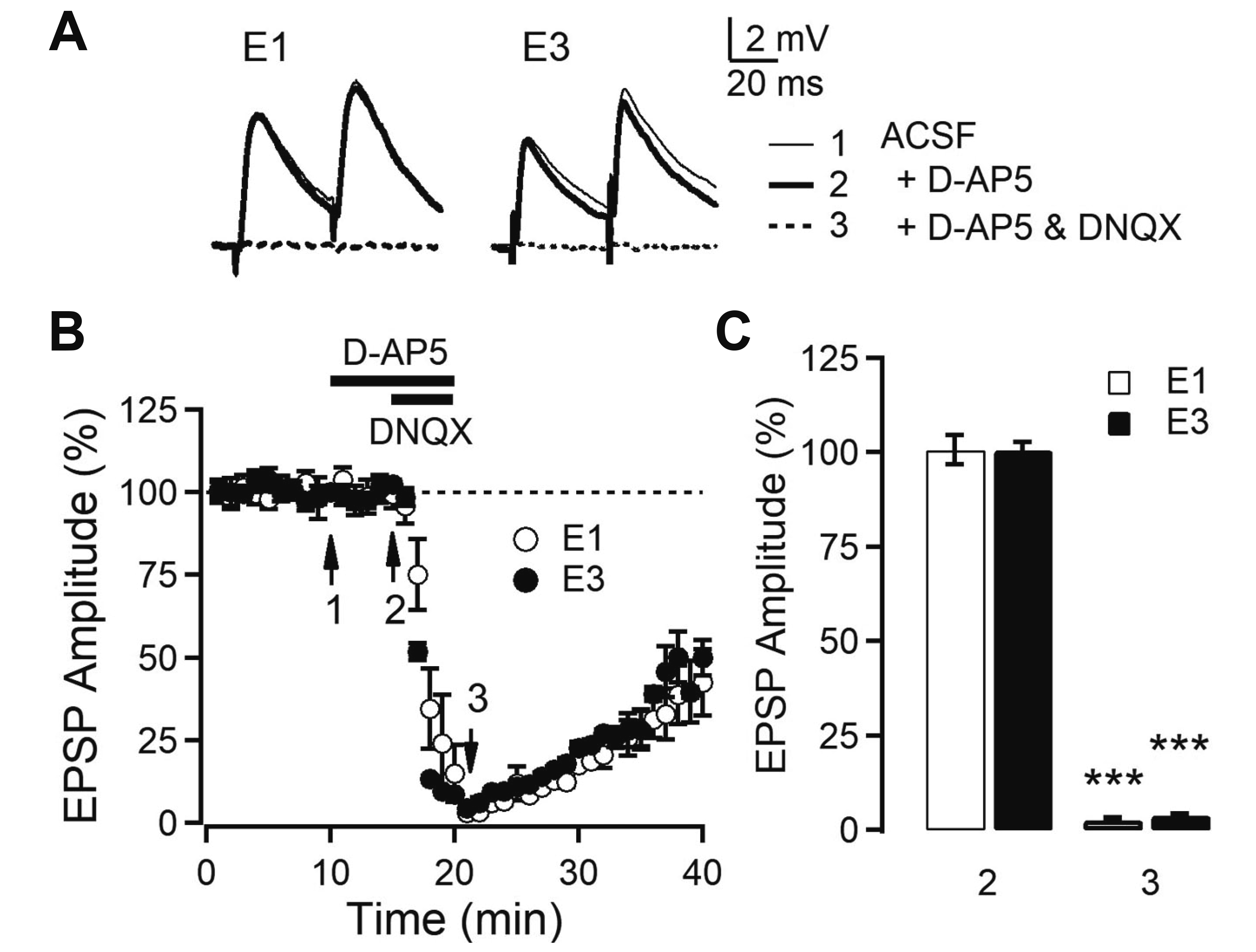 | Fig. 2AMPA receptor-mediated synaptic transmission in Layer 2/3 (L2/3) pyramidal neurons of orbitofrontal cortex.(A) Representative EPSPs evoked by L1 and L3 stimulation (E1 and E3, respectively). The average EPSP waveforms recorded during 1 min at the time indicated by numbered arrows in B. (B) Time course of EPSP amplitude with the application of blockers. The amplitude of first EPSPs evoked by paired-pulse stimulation in the presence and absence of D-AP5 (50 μM) and/or DNQX (20 μM) in bath solution. (C) Summary plot of EPSP amplitude by E1 (n = 6) and E3 (n = 5). ***p < 0.001 compared with baseline. EPSP, excitatory postsynaptic potential; D-AP5, d-(-)-2-amino-5-phosphonopentanoic acid; DNQX, 6,7-dinitroquinoxaline-2,3-dione disodium salt; ACSF, artificial cerebrospinal fluid.
|
Layer-specific serotonergic modulation of glutamatergic transmission in L2/3 PyNs
 | Fig. 3Serotonergic induction of long-term depression (LTD) in the Layer 2/3 (L2/3) pyramidal neurons of orbitofrontal cortex.(A) Time course of average EPSP amplitude evoked by L1 and L3 stimulation. Serotonin (5-HT; 10 μM, 10 min) was applied in bath solution (closed symbols: n = 9). In some experiments electrical stimulation was omitted during the application of 5-HT and until 10 min after washout (open symbols: n = 6). Insets show average EPSPs taken at the time indicated from a representative experiment. (B) Left panels show individual data and averages (thick solid lines). Right panels show the change in paired-pulse ratio (PPR) of closed symbols of the left panels. **p < 0.01 compared with the baseline and ##p < 0.01. (C) Concentration response plot of EPSP amplitude with L3 stimulation. The curve was fitted by sigmoid function (EC50 = 3.57 μM). ***p < 0.001 compared with the normal ACSF. EPSP, excitatory postsynaptic potential; ACSF, artificial cerebrospinal fluid.
|
5-HT2 receptor mediates serotonergic LTD induction
 | Fig. 45-HT2 receptor mediates the effect of 5-HT induction of long-term depression (LTD).Summary plots of average EPSP amplitude with L1 (A) and L3 (B) stimulation of Layer 2/3 (L2/3) pyramidal neurons of orbitofrontal cortex. 5-HT antagonists were applied into the bath throughout the experiments. 5-HT (n = 9), α-me-5-HT (n = 6), 8-OH-DPAT (n = 6), mCPBG (n = 7), α-me-5-HT + ketanserin (n = 6), ketanserin (n = 7), NAN-190 (n = 5), and ondansetron (n = 6). 5-HT, serotonin; 8-OH-DPAT, 8-hydroxy-2-dipropylaminotetralin hydrobromide; mCPBG, 1-(m-chlorophenyl) biguanide hydrochloride; α-me-5-HT, α-methyl-5-hydroxytryptamine; EPSP, excitatory postsynaptic potential. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the baseline.
|
LTD induction is dependent on intracellular Ca2+ via NMDA receptors
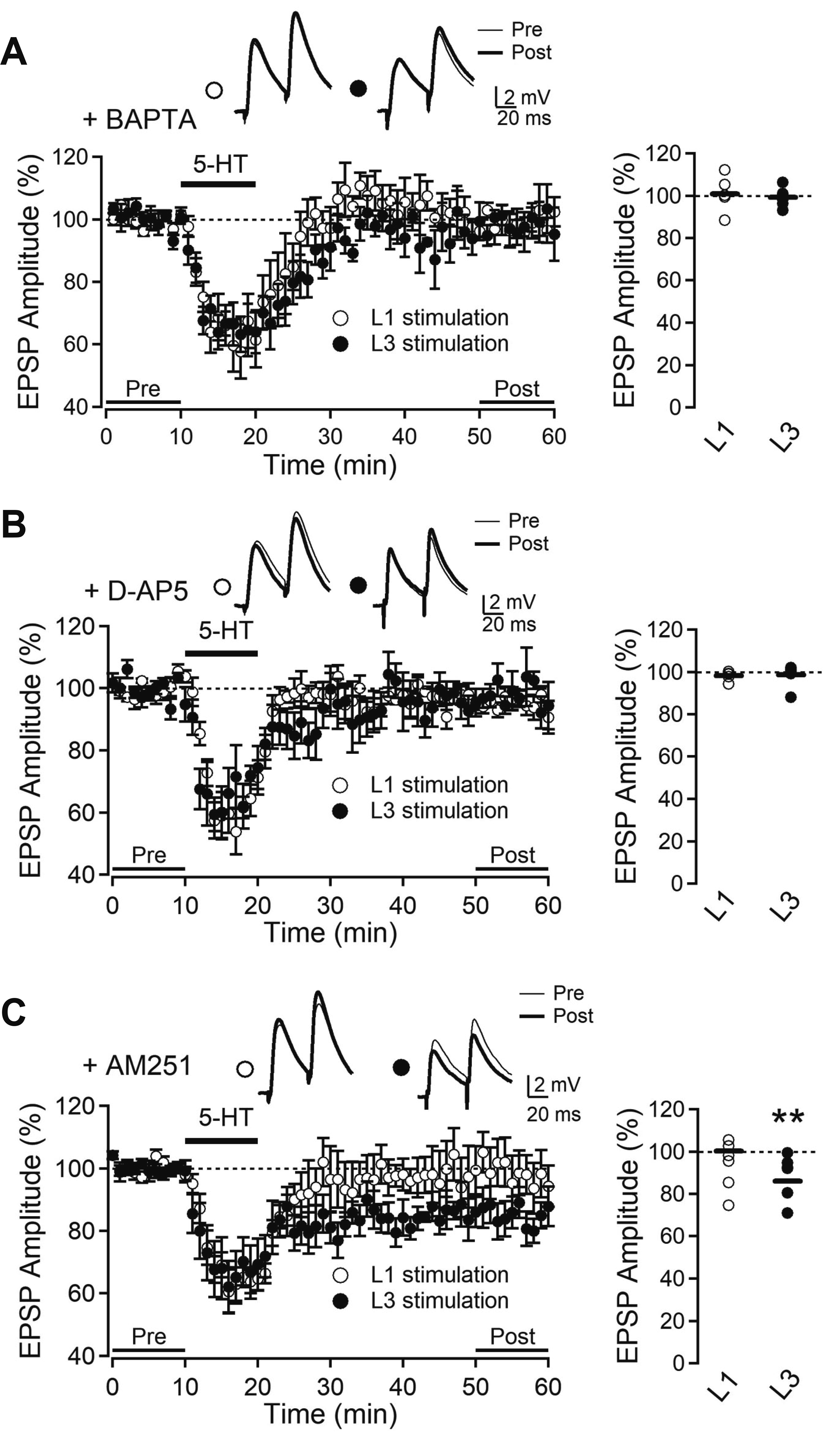 | Fig. 5Serotonergic long-term depression (LTD) is dependent on intracellular Ca2+ and NMDA receptors.(A) Time course of average EPSP amplitude in the presence of intracellular BAPTA (10 mM) with L1 stimulation (open circle, n = 6) and L3 stimulation (closed circle, n = 6). (B) Time course of average EPSP amplitude with bath application of D-AP5 (50 μM) evoked by L1 stimulation (open circle, n = 5) and L3 stimulation (closed circle, n = 6). (C) Time course of average EPSP amplitude with bath application of AM251 (5 μM) evoked by L1 stimulation (open circle, n = 5) and L3 stimulation (closed circle, n = 6). Insets show average EPSPs taken at the time indicated from a representative experiment. Right panels show EPSP amplitude in individual experiments and the average (solid line). EPSP, excitatory postsynaptic potential; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid; D-AP5, d-(-)-2-amino-5-phosphonopentanoic acid; AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide. **p < 0.01 compared with baseline (pre).
|
Cholinergic induction of LTD
 | Fig. 6Cholinergic induction of long-term depression (LTD).(A)Time course of average EPSP amplitude evoked by L1 and L3 stimulation. Carbachol (CCh, 10 μM) was applied in bath solution (closed circle: n = 9, closed triangle: n = 6). Atropine (10 μM) was applied to the bath solution throughout the experiment (open circle: n = 7, open triangle: n = 5). Insets show average EPSPs taken at the time indicated from a representative experiment. (B) Left panels show individual data and averages (thick solid lines). Right panels show the change in paired-pulse ratio (PPR) of closed symbols of the left panels. EPSP, excitatory postsynaptic potential. **p < 0.01 compared with the baseline, #p < 0.05, ##p < 0.01.
|




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download