INTRODUCTION
METHODS
Chemicals
Animals and experimental design
Measurement of paw volume and arthritic score
Analysis of serum hematology and biochemistry
ELISA assay for inflammatory cytokine analysis
Histopathological examination
Statistical analysis
RESULTS
D-carvone improves body weight, paw volume, arthritic score, and organ index of CFA-induced arthritic rats
Table 1
Values expressed as mean ± standard error of mean (n = 8) of final body weight and organ index of CFA-induced rats and D-carvone administered rats. Group I = Normal control; Group II = CFA-induced arthritis model; Group III = D-carvone 30 mg/kg b.w. + CFA; Group IV = D-carvone 60 mg/kg b.w. + CFA; Group V = Indomethacin positive control + CFA. CFA, complete Freund’s adjuvant; b.w., body weight. Superscript ‘a’ indicates significant difference (p < 0.05) from Group I, superscript ‘b’ indicates significant difference (p < 0.05) from Group II, superscript ‘c’ indicates significant difference (p < 0.05) from Group III, by one-way ANOVA followed by Tukey’s multiple comparisons test.
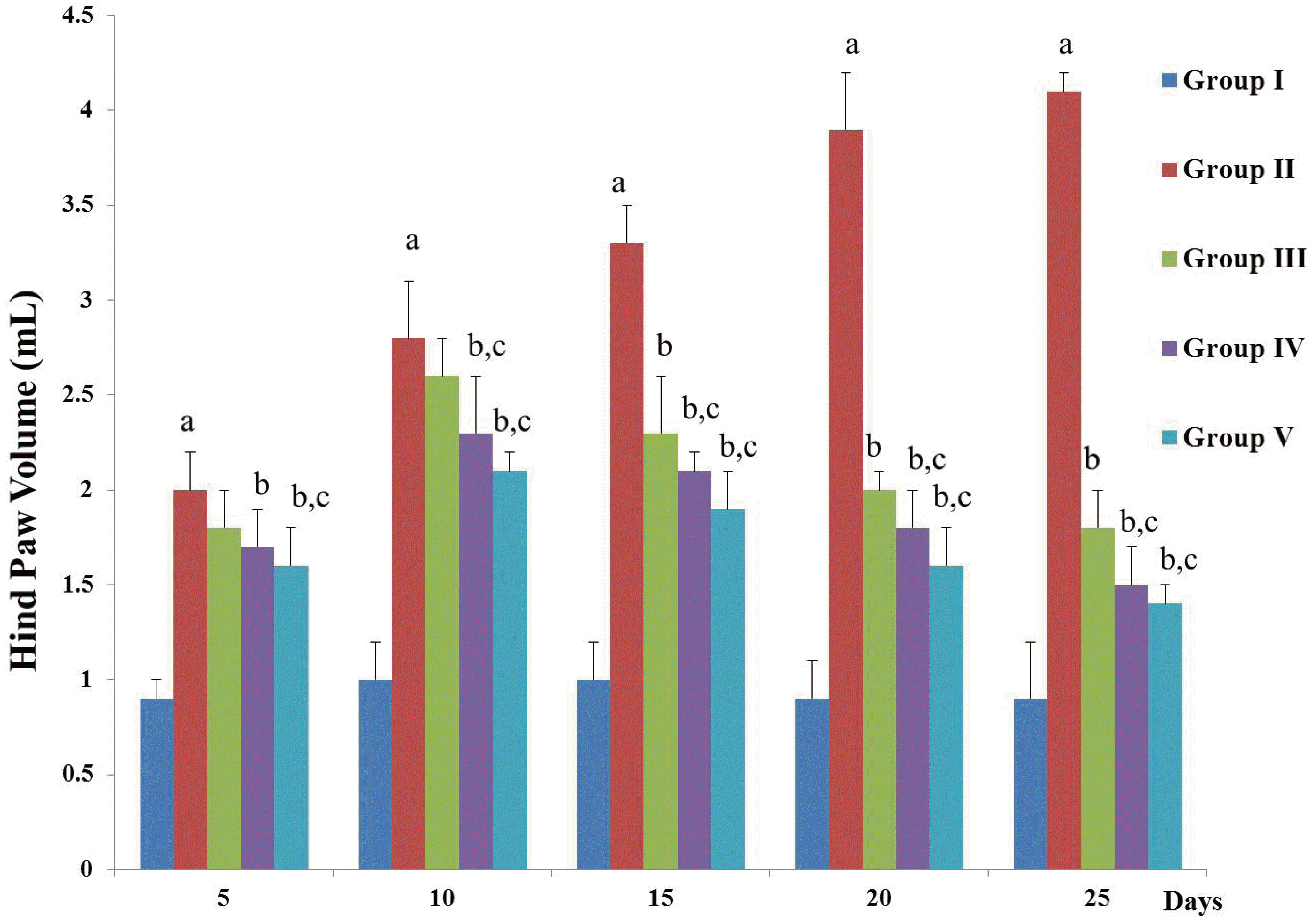 | Fig. 1D-carvone reduces the hind paw volume of CFA-induced arthritic rats.Values expressed as mean ± standard error of mean of (n = 8) rats. Group I = Normal control; Group II = CFA-induced arthritis model; Group III = D-carvone 30 mg/kg b.w. + CFA; Group IV = D-carvone 60 mg/kg b.w. + CFA; Group V = Indomethacin positive control + CFA. CFA, complete Freund’s adjuvant; b.w., body weight. Superscript ‘a’ indicates significant difference (p < 0.05) from Group I, superscript ‘b’ indicates significant difference (p < 0.05) from Group II, superscript ‘c’ indicates significant difference (p < 0.05) from Group III, by one-way ANOVA followed by Tukey’s multiple comparisons test.
|
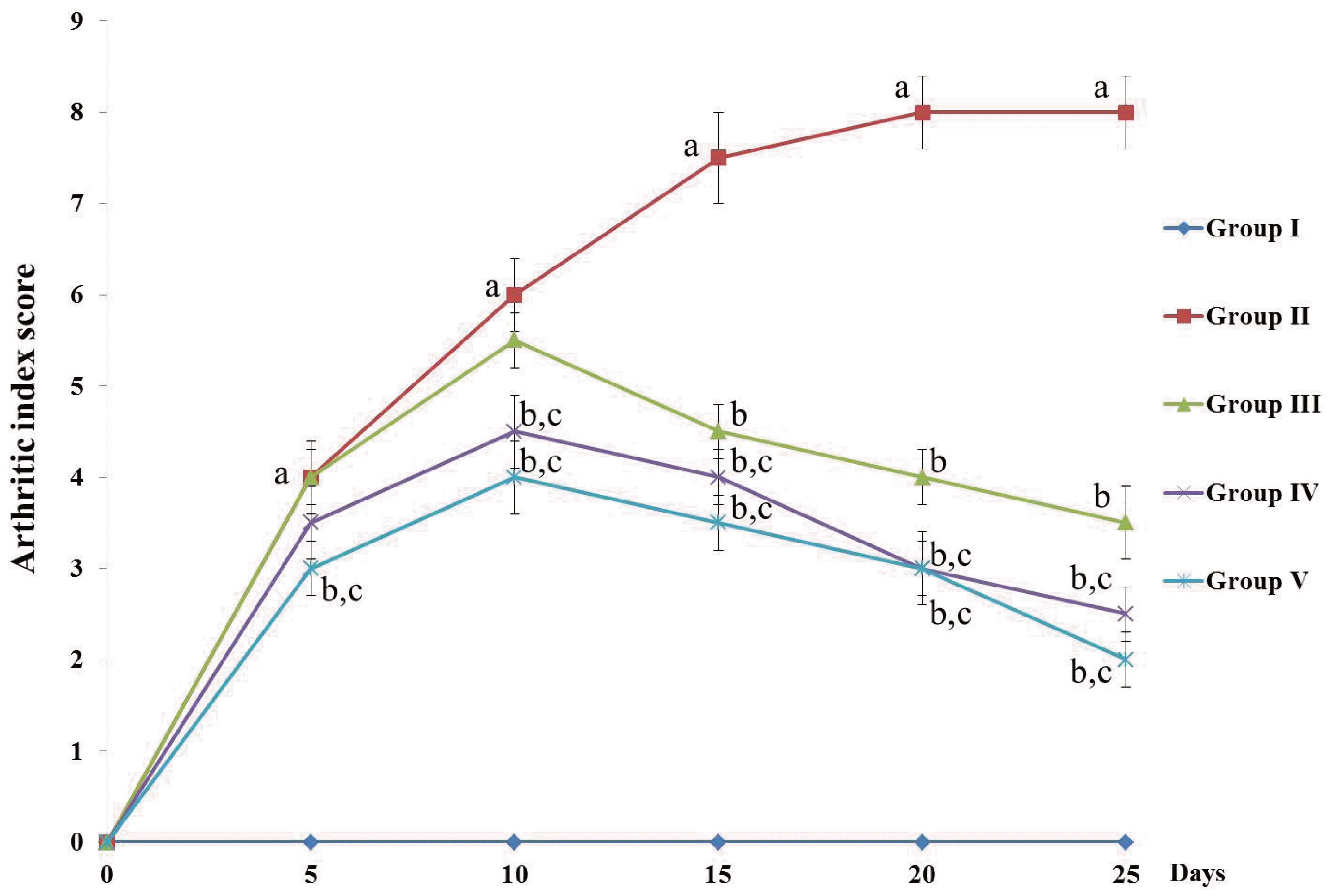 | Fig. 2D-carvone alters the arthritic score of CFA-induced inflammation in rats.Values expressed as mean ± standard error of mean of (n = 8) rats. The arthritic score was ranged from 0 (normal paw with no swelling or erythema)–4 (severe deformity of the paw with swelling and erythema) for a maximum score of 8 for both hind paws. Group I = Normal control; Group II = CFA-induced arthritis model; Group III = D-carvone 30 mg/kg b.w. + CFA; Group IV = D-carvone 60 mg/kg b.w. + CFA; Group V = Indomethacin positive control + CFA. CFA, complete Freund’s adjuvant; b.w., body weight. Superscript ‘a’ indicates significant difference (p < 0.05) from Group I, superscript ‘b’ indicates significant difference (p < 0.05) from Group II, superscript ‘c’ indicates significant difference (p < 0.05) from Group III, by one-way ANOVA followed by Tukey’s multiple comparisons test.
|
Effects of D-carvone on CFA-induced hematological changes in rats
Table 2
Values expressed as mean ± standard error of mean (n = 8) of hematological changes due to D-carvone administration in CFA-induced rats. Group I = Normal control; Group II = CFA-induced arthritis model; Group III = D-carvone 30 mg/kg b.w. + CFA; Group IV = D-carvone 60 mg/kg b.w. + CFA; Group V = Indomethacin positive control + CFA. CFA, complete Freund’s adjuvant; b.w., body weight; WBC, white blood cells; RBC, red blood cells; Hb, hemoglobin. Superscript ‘a’ indicates significant difference (p < 0.05) from Group I, superscript ‘b’ indicates significant difference (p < 0.05) from Group II, superscript ‘c’ indicates significant difference (p < 0.05) from Group III, by one-way ANOVA followed by Tukey’s multiple comparisons test.
D-carvone attenuates serum biochemical changes in CFA-induced arthritic rats
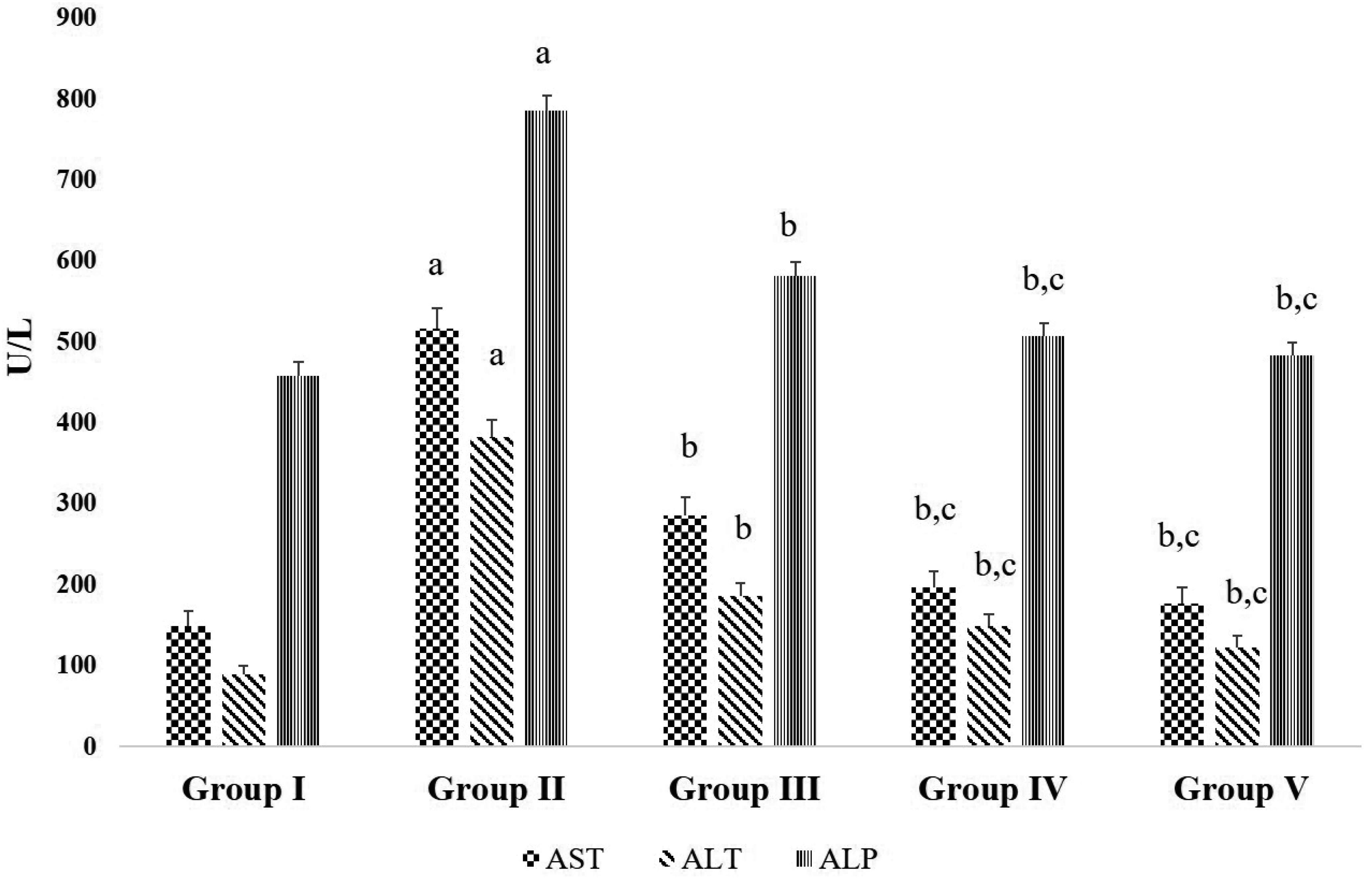 | Fig. 3Effects of D-carvone on serum liver markers in CFA-induced arthritic rats.Values expressed as mean ± standard error of mean (n = 8) of hematological changes due to D-carvone administration in CFA-induced rats. Group I = Normal control; Group II = CFA-induced arthritis model; Group III = D-carvone 30 mg/kg b.w. + CFA; Group IV = D-carvone 60 mg/kg b.w. + CFA; Group V = Indomethacin positive control + CFA. CFA, complete Freund’s adjuvant; b.w., body weight; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase. Superscript ‘a’ indicates significant difference (p < 0.05) from Group I, superscript ‘b’ indicates significant difference (p < 0.05) from Group II, superscript ‘c’ indicates significant difference (p < 0.05) from Group III, by one-way ANOVA followed by Tukey’s multiple comparisons test.
|
 | Fig. 4Effects of D-carvone on oxidative stress in CFA-induced arthritic rats.Values expressed as mean ± standard error of mean (n = 8) of lipid peroxidation, enzymatic and non-enzymatic antioxidants. Group I = Normal control; Group II = CFA-induced arthritis model; Group III = D-carvone 30 mg/kg b.w. + CFA; Group IV = D-carvone 60 mg/kg b.w. + CFA; Group V = Indomethacin positive control + CFA. CFA, complete Freund’s adjuvant; b.w., body weight; MDA, malondialdehyde; GSH, reduced glutathione; SOD, superoxide dismutase; CAT, catalase. Superscript ‘a’ indicates significant difference (p < 0.05) from Group I, superscript ‘b’ indicates significant difference (p < 0.05) from Group II, superscript ‘c’ indicates significant difference (p < 0.05) from Group III, by one-way ANOVA followed by Tukey’s multiple comparisons test.
|
Effects of D-carvone on CFA-induced changes of inflammatory cytokines
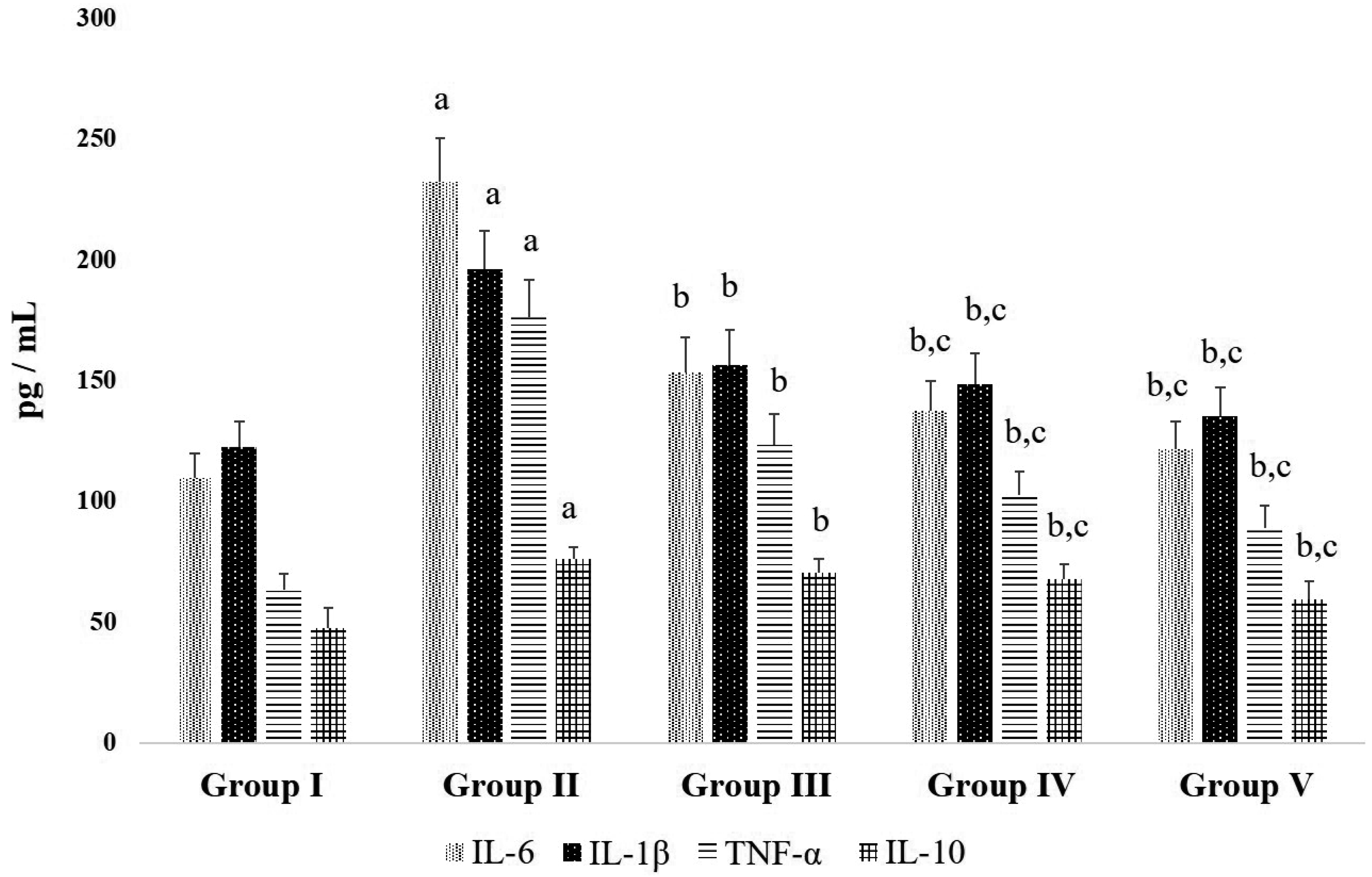 | Fig. 5Effects D-carvone on inflammatory cytokines in CFA-induced arthritic rats.Values expressed as mean ± standard error of mean (n = 8) of serum inflammatory cytokines. Group I = Normal control; Group II = CFA-induced arthritis model; Group III = D-carvone 30 mg/kg b.w. + CFA; Group IV = D-carvone 60 mg/kg b.w. + CFA; Group V = Indomethacin positive control + CFA. CFA, complete Freund’s adjuvant; b.w., body weight; IL, interleukin; TNF, tumor necrosis factor. Superscript ‘a’ indicates significant difference (p < 0.05) from Group I, superscript ‘b’ indicates significant difference (p < 0.05) from Group II, superscript ‘c’ indicates significant difference (p < 0.05) from Group III, by one-way ANOVA followed by Tukey’s multiple comparisons test.
|
Histopathological modifications by D-carvone in CFA-induced arthritic rats
 | Fig. 6Modulating effect of D-carvone on the hind paw histopathology of CFA-induced rats.(A) Hind paw sections of arthritis model group II shows severe signs of articular cartilage damage, infiltration of inflammatory cells, synovial hyperplasia, and bone marrow degeneration. Administration of D-carvone in rats of group III and IV ameliorated the CFA-induced arthritic inflammation and changes. Positive control indomethacin group V also exhibited positive changes against CFA-induced arthritic damage. Arrow indicates articular cartilage erosion. Scale bar = 500 µm; H&E staining at 100× magnification. (B) Total Mankin’s score for articular injuries and cellular irregularities. Group I = Normal control; Group II = CFA-induced arthritis model; Group III = D-carvone 30 mg/kg b.w. + CFA; Group IV = D-carvone 60 mg/kg b.w. + CFA; Group V = Indomethacin positive control + CFA. CFA, complete Freund’s adjuvant; b.w., body weight. Superscript ‘a’ indicates significant difference (p < 0.05) from Group I, superscript ‘b’ indicates significant difference (p < 0.05) from Group II, superscript ‘c’ indicates significant difference (p < 0.05) from Group III, by one-way ANOVA followed by Tukey’s multiple comparisons test.
|




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download