INTRODUCTION
METHODS
Animals and brain slice preparation
Whole-cell patch-clamp recording
Chemicals and statistics
RESULTS
Borneol induces repeatable inward currents on SG neurons
 | Fig. 2Borneol-induced inward currents are repeatable on the SG neurons of the Vc.(A) A representative trace showing the repeatable inward currents by borneol (0.3 mM) under high chloride pipette solution. (B) The before and after plot shows no significant difference in the mean inward currents between the 1st and the 2nd applications of 0.3 mM borneol (n = 17, paired t-test, p > 0.05). NS implicates not significant.
|
Borneol directly acts on SG neurons
 | Fig. 3Direct action of borneol on the SG neurons of the Vc.(A) A representative current trace showing no effect on the borneol-induced response by tetrodotoxin (TTX, 0.5 µM), a voltage-sensitive Na+ channel blocker under high chloride pipette solution. (B) The before and after plot shows no significant difference in the mean borneol-induced inward currents between the absence and the presence of tetrodotoxin (n = 13, paired t-test, p > 0.05). NS implicates not significant.
|
Borneol activates GABAA receptor and/or glycine receptor
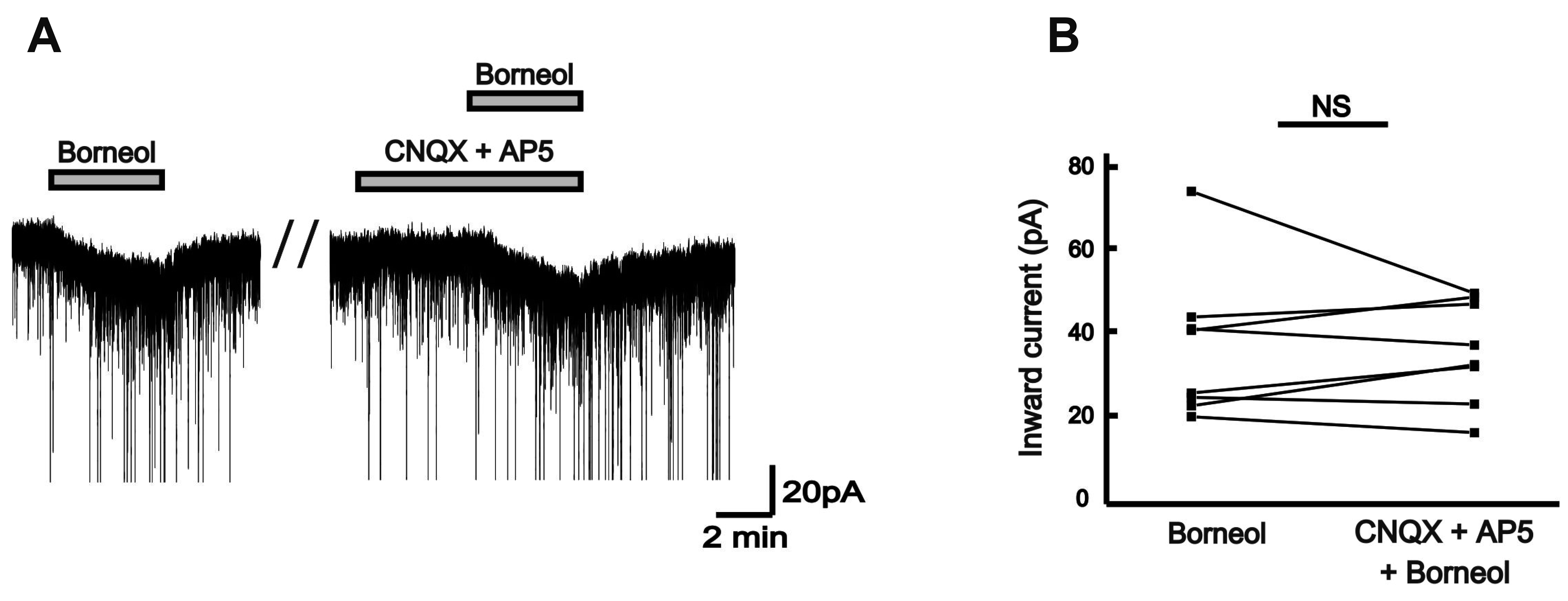 | Fig. 4Borneol-induced action is not mediated by ionotropic glutamate receptor activation.(A) A representative current trace showing no effect on the borneol-induced response by 6-cyano-7-nitro-quinoxaline-2,3-dione (CNQX) (10 µM) and DL-2-amino-5-phosphonopentanoic acid (AP5) (20 µM), ionotropic glutamate receptor antagonists. (B) The before and after plot shows no significant difference in the borneol-induced inward currents between the absence and the presence of CNQX and AP5 (n = 8, paired t-test, p > 0.05). NS implicates not significant.
|
 | Fig. 5Borneol activates GABAA receptor on the SG neurons of the Vc.(A) A representative current trace showing the inhibition of borneol-induced inward current by picrotoxin (50 µM), a GABAA receptor antagonist. (B) The before and after plot shows significant inhibition of mean inward currents by borneol in the presence of picrotoxin (n = 10, paired t-test, ***p < 0.001).
|
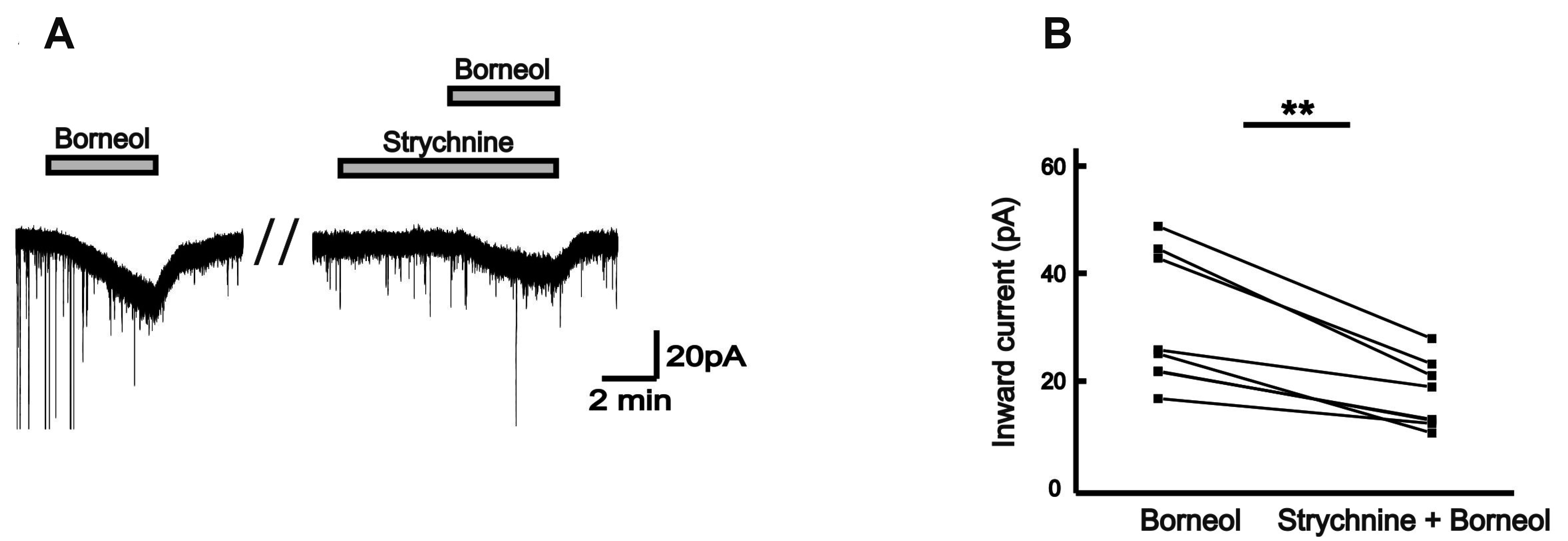 | Fig. 6Borneol activates glycine receptor on the SG neurons of the Vc.A representative current trace showing the inhibition of the borneol-induced inward currents by strychnine (2 µM), a glycine receptor antagonist. (B) The before and after plot shows significant inhibition of mean inward currents by borneol in the presence of strychnine (n = 7, paired t-test, **p < 0.01).
|
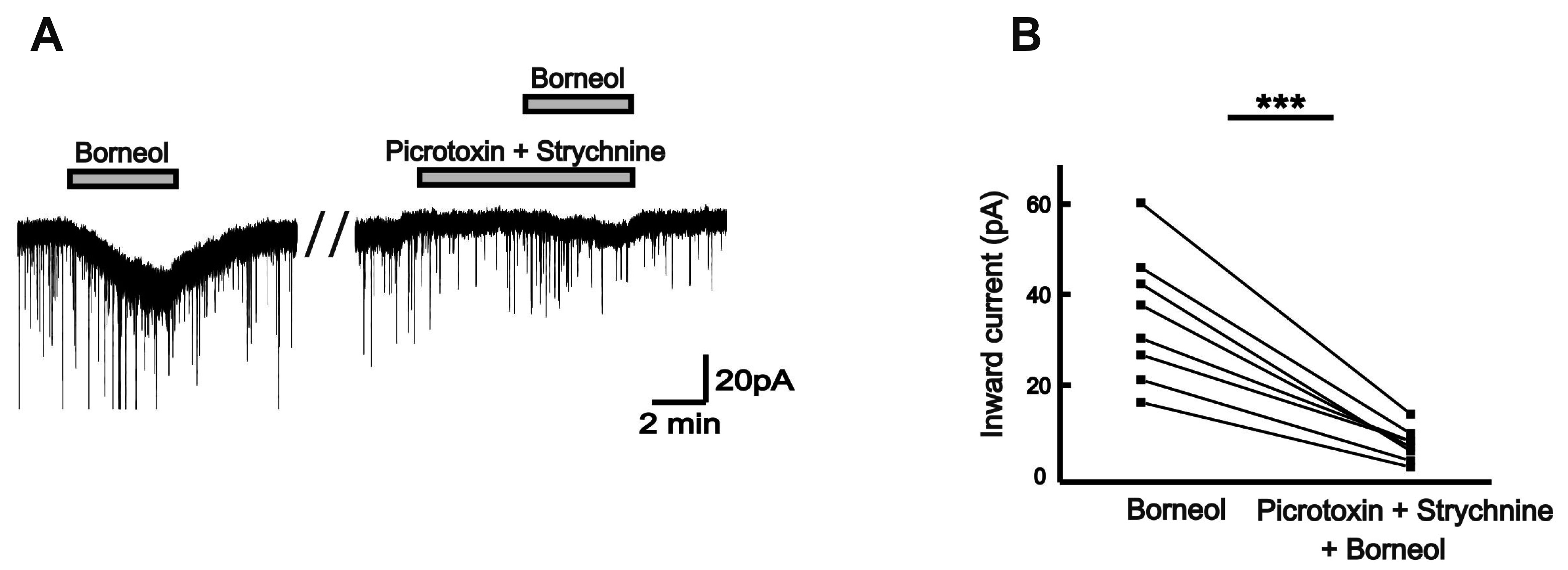 | Fig. 7GABA- and/or glycine-mimetic actions of borneol.(A) A representative current trace showing inhibition of the borneol-induced inward currents by both picrotoxin (50 µM) and strychnine (2 µM). (B) The before and after plot shows significant inhibition of mean inward currents by borneol in the presence of picrotoxin and strychnine (n = 8, paired t-test, ***p < 0.001).
|
Borneol enhances GABA-mediated actions
 | Fig. 8Borneol enhances on GABA-induced responses but not glycine-induced responses.(A) A representative current trace showing the increase of the GABA-induced response by borneol. (B) The before and after plot shows significant increase of mean inward current by GABA (30 µM) in the presence of 0.2 mM borneol (n = 5, paired t-test, *p < 0.05). (C) A representative current trace showing no change of the glycine-induced response by borneol. (D) The before and after plot shows no significant change in the mean inward currents by glycine (30 µM) in the presence of 0.2 mM borneol (n = 8, paired t-test, p > 0.05). NS implicates not significant.
|
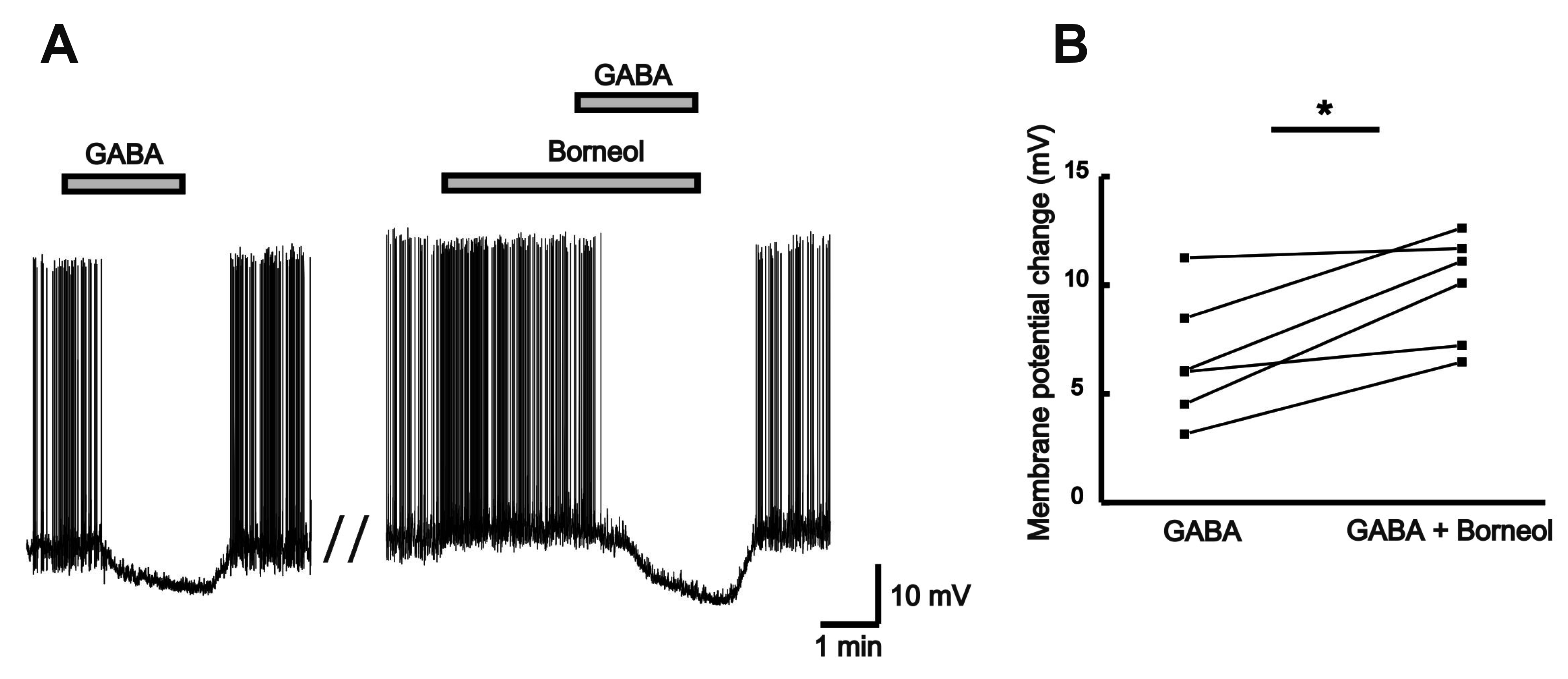 | Fig. 9Borneol enhances GABA-induced hyperpolarization.(A) A representative trace showing the membrane hyperpolarization induced by GABA (30 µM) alone and GABA in the presence of borneol (0.3 mM) in the current-clamp mode. (B) The before and after plot shows significant enhancement of mean membrane potential change by GABA in the absence and the presence of borneol (n = 6, paired t-test, *p < 0.05).
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download