INTRODUCTION
METHODS
Cell culture and reagents
Plasmid constructs and oligonucleotides
Transfection and luciferase activity assay
Western blotting
Apoptosis assay
RT-PCR
Statistical analysis
RESULTS
Expression of oncogenic H-Ras inhibits rat mdr1b expression
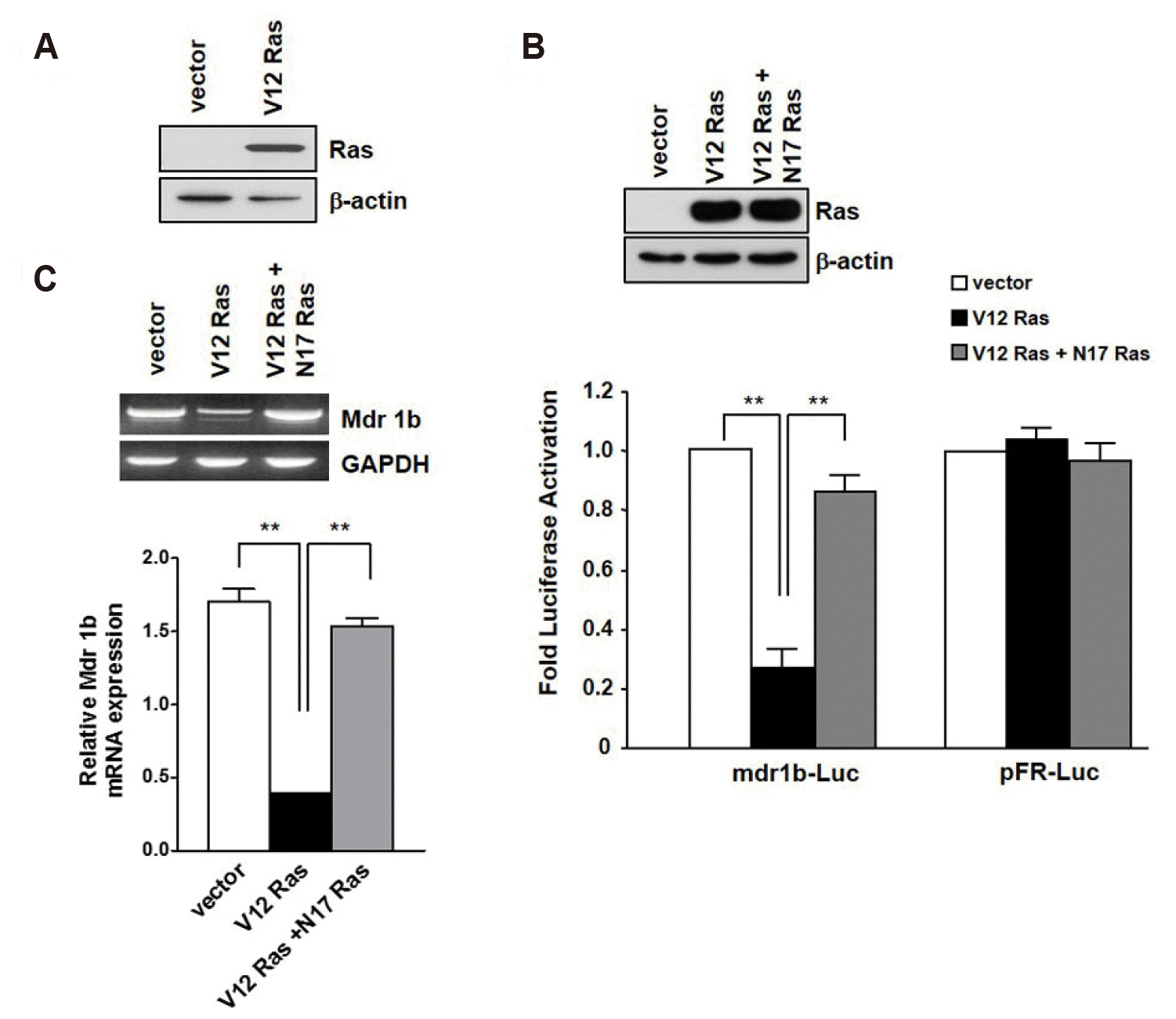 | Fig. 1Effect of oncogenic H-Ras overexpression on mdr1b expression.(A) The levels of overexpressed V12-Ras was analyzed by Western blotting. (B) The pcDNA3-NIH3T3 (vector) and V12-Ras-NIH3T3 (V12-Ras) cells were cotransfected with either mdr1b-Luc or pFR-Luc and pRL-Luc. The luciferase activities were measured 24 h after transfection, as described under “Methods”. Transfection with the pRL-Luc plasmid was used to normalize the transfection. The level of luciferase activation is presented relative to the activity obtained from the transfection of mdr1b-Luc into the pcDNA3-NIH3T3 cells, whose value was placed at 1.0. To block the Ras signaling pathway, N17-Ras-pcDNA3 (N17-Ras) was transiently transfection into V12-Ras-NIH3T3 cells, after which cells were transfected with mdr1b-Luc. Each data bar represents the mean of five observations from three independent experiments; the error bars indicate ± standard deviation. (C) RT-PCR analysis of mdr1b gene expression in pcDNA3-NIH3T3 (vector), V12-Ras-NIH3T3 (V12) and N17-Ras transfecting V12-Ras-NIH3T3 cells (V12+N17). The total RNA (2 µg) was reverse transcribed using reverse transcriptase. cDNA amplification was carried out using taq-polymerase over 30 cycles. The PCR products were separated on 1.5% agarose gels, stained with ethidium bromide. Bar graphs quantify the amount of mdr1b mRNA by Image J software. **p < 0.01.
|
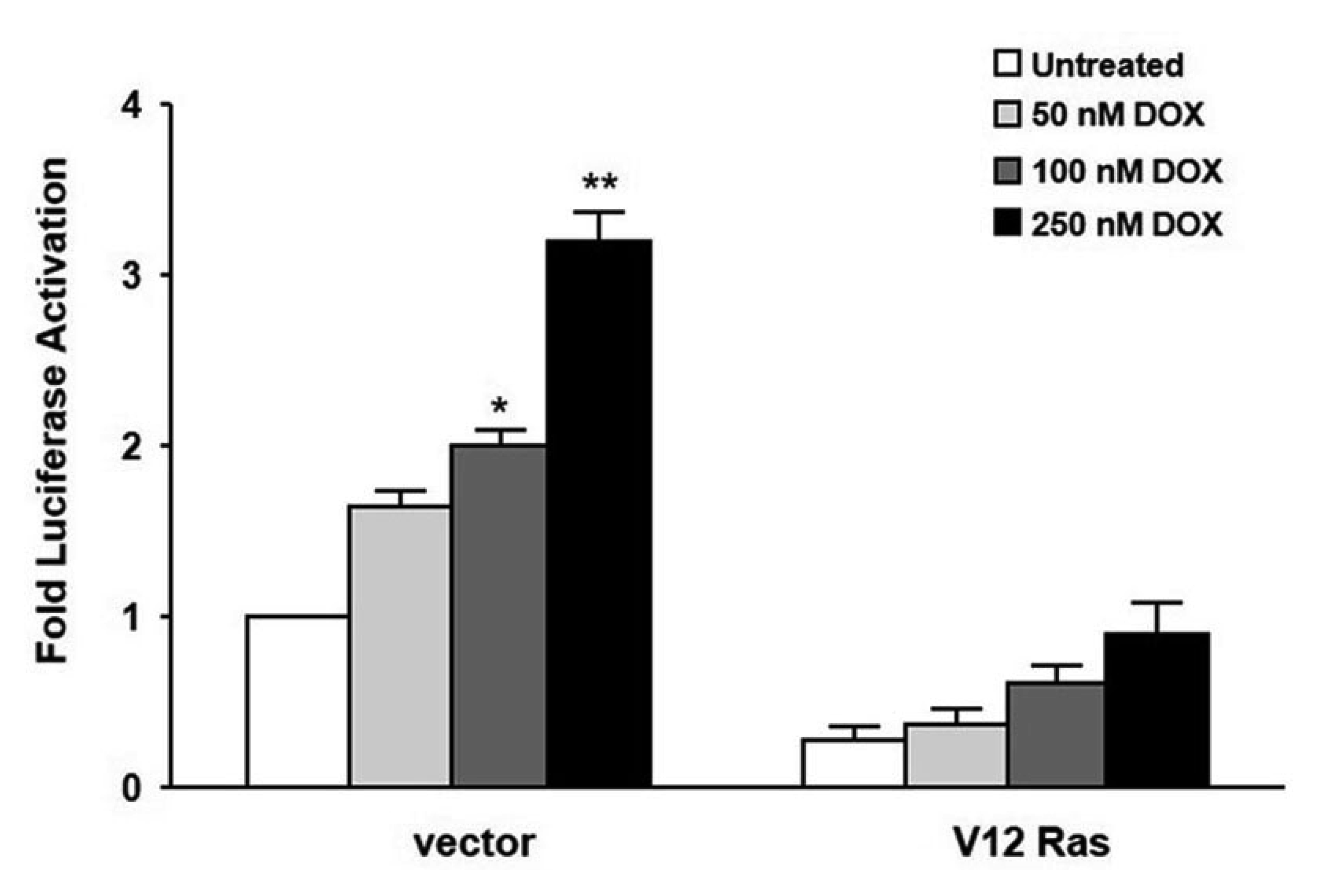 | Fig. 2pcDNA3-NIH3T3 (vector) and V12-Ras-NIH3T3 (V12-Ras) cells were cotransfected with either the mdr1b-Luc reporter plasmid or the pFR-Luc and pRL-Luc plasmid.Four hours after transfection, the cells were treated with different doxorubicin (DOX) concentrations for additional 24 h, and the luciferase activities were determined as described in Fig. 1B. Each data bar represents the mean of five observations from three independent experiments; the error bars indicate ± standard deviation. *p < 0.05 vs. untreated. **p < 0.01.
|
Intracellular ROS production by the expression of oncogenic Ras inhibits mdr1b expression
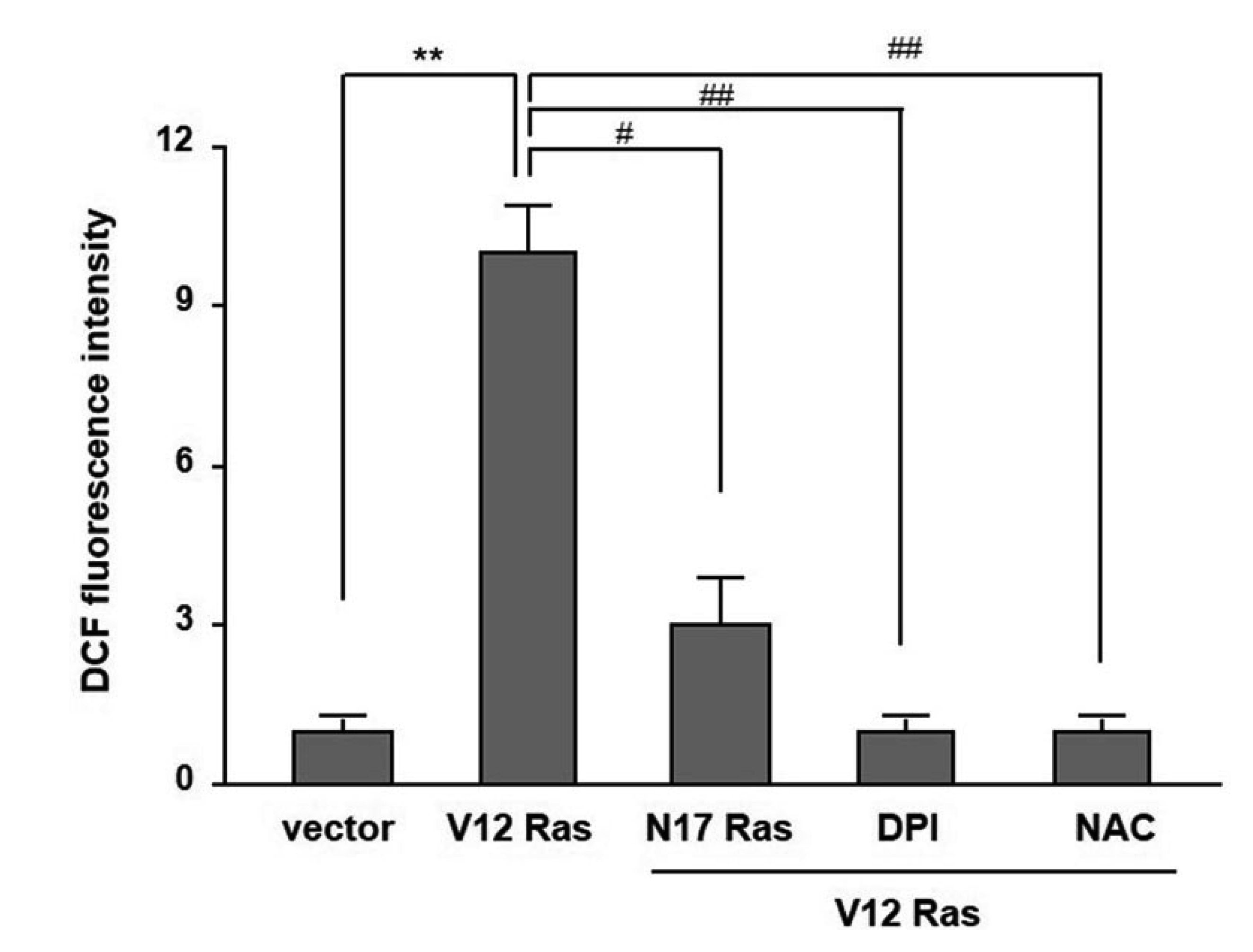 | Fig. 3Reactive oxygen species (ROS) production in the pcDNA3-NIH3T3 (vector) and V12-Ras-NIH3T3 (V12-Ras) cells.The cells were pretreated with 20 mM N-acetylcysteine (NAC) or 500 nM diphenylene iodonium (DPI), or transiently transfected with N17-Ras-pcDNA3 (N17-Ras). They were then incubated with DCFHDA, and a ROS assay was carried out as describe in “Methods”. Each point is the average of multiple independent experiments; the error bars represent ± standard deviation. **p < 0.01 vs. vector, #p < 0.05, ##p < 0.001 vs. V12-Ras.
|
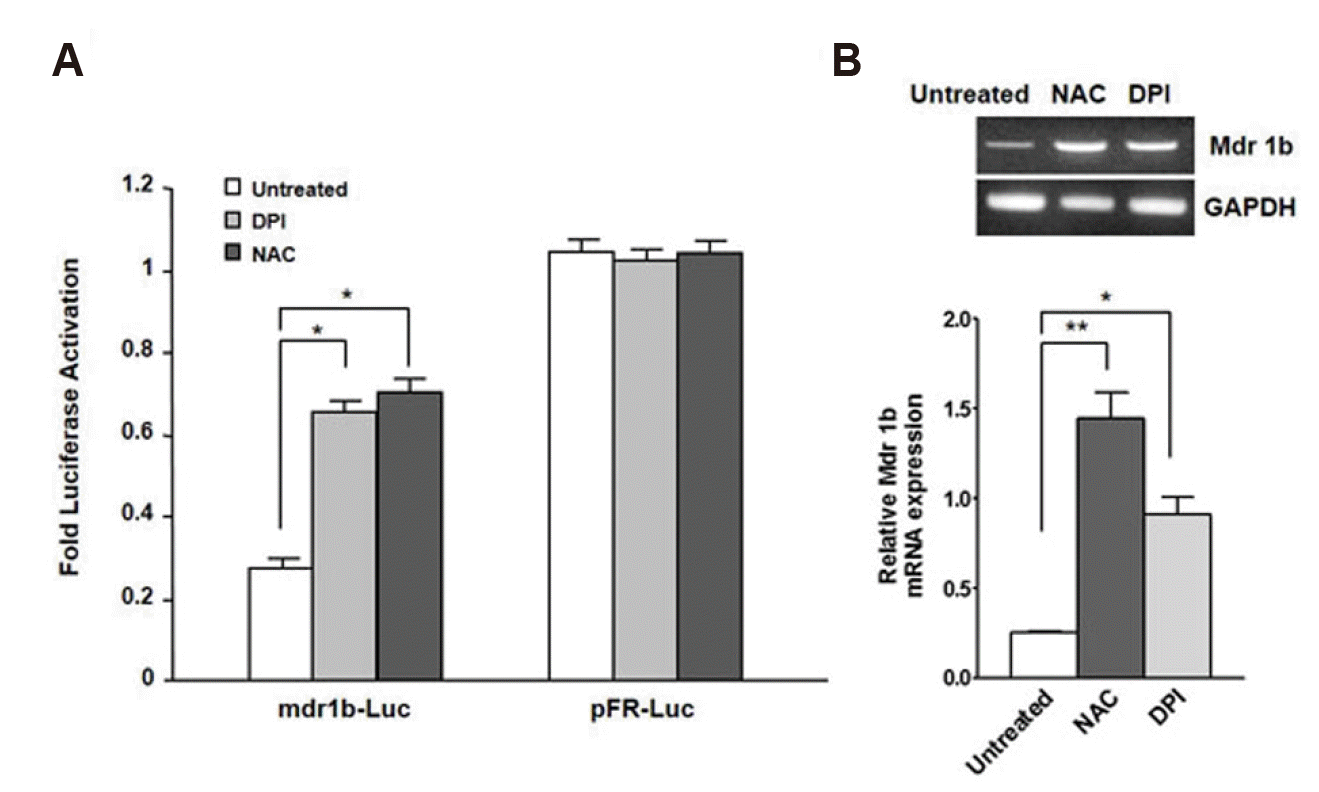 | Fig. 4Effect of N-acetylcysteine (NAC) and diphenylene iodonium (DPI) on basal level of mdr1b expression.(A) The V12-Ras-NIH3T3 (V12-Ras) cells were pretreated with either 20 mM NAC or 500 nM DPI for 12 h, and then cotransfected with either mdr1b-Luc or pFR-Luc and pRL-Luc. The luciferase activities were the luciferase activity was determined as described in Fig. 1A. Each point is the average of multiple independent experiments; the error bars represent ± standard deviation. (B) RT-PCR analysis of the mdr1b gene expression level in V12-Ras-NIH3T3 cells with NAC or DPI. The cells were pretreated with either NAC or DPI for 12 h. RT-PCR was performed with the total RNA extract (20 µg) and the PCR products were separated on 1.5% agarose gels, and stained with ethidium bromide. *p < 0.05, **p < 0.01 vs. untreated.
|
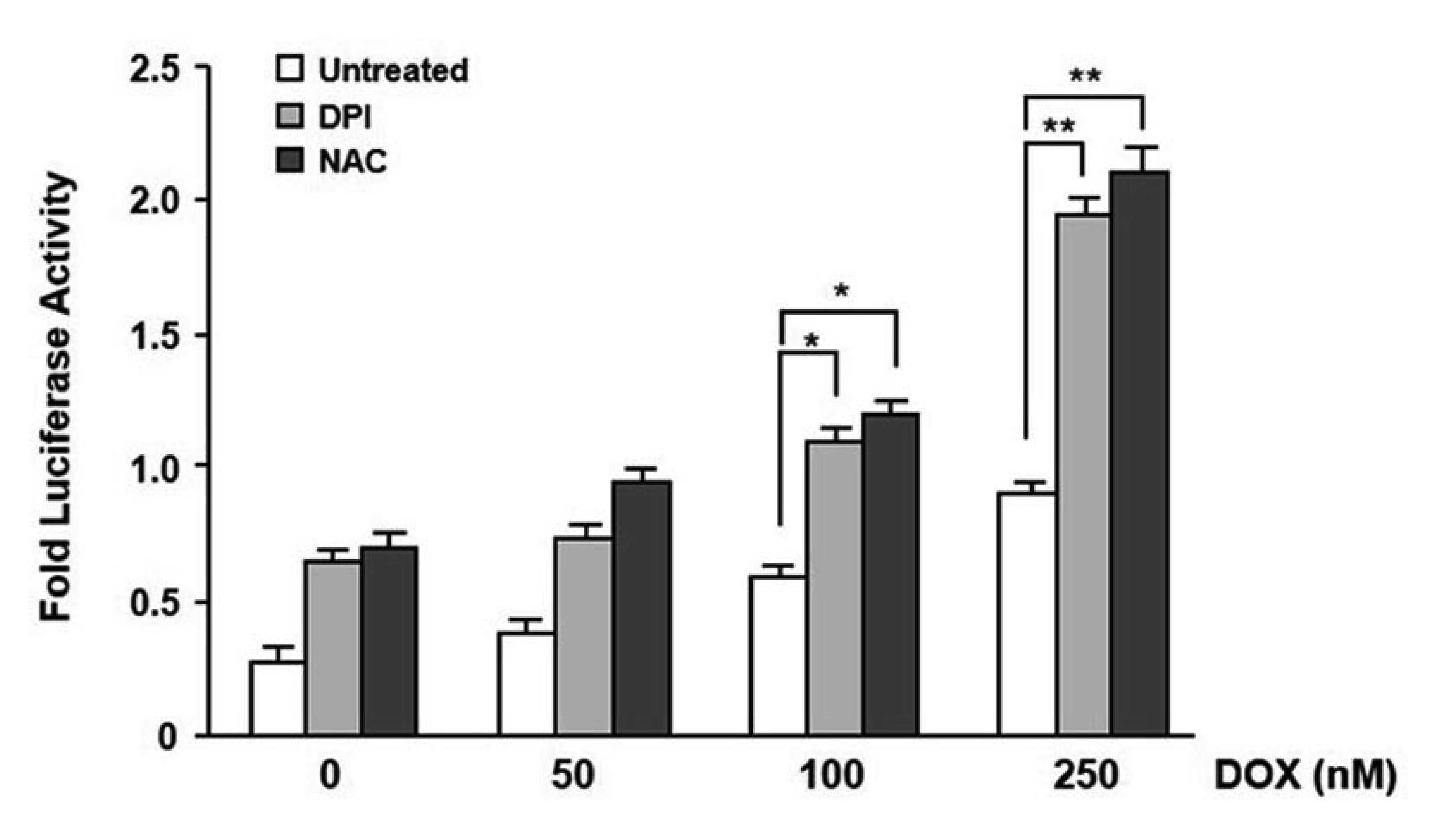 | Fig. 5Effect of N-acetylcysteine (NAC) and diphenylene iodonium (DPI) on the induction level of mdr1b expression by doxorubicin (DOX).The V12-Ras-NIH3T3 cells were pretreated with 20 mM NAC or 500 nM DPI for 12 h and then cotransfected with either mdr1b-Luc or pFR-Luc and pRL-Luc. Four hours after transfection, the cells were treated with 50, 100, or 250 nM doxorubicin for 24 h, after which the luciferase activities were determined as described in Fig. 1A. Each point is the average of multiple independent experiments; the error bars represent ± standard deviation. *p < 0.05 vs. untreated. **p < 0.01.
|
Involvement of ERK signal transduction in the downregulation of mdr1b expression
 | Fig. 6Effect of ERK signaling pathway in the regulation of mdr1b expression.(A) pcDNA3-NIH3T3 (vector) and V12-Ras-NIH3T3 (V12-Ras) cells were pretreated with either DMSO or the indicated compounds, at the following concentrations: 50 µM PD98059, 20 µM U0126, 10 mM NAC, and 500 nM DPI. Subsequently, the cells were cotransfected with either the Gal4-Elk1 or pFR-Luc plasmid and pRL-CMV. The luciferase activities were then measured 24 h after transfection determined as described in Fig. 1A. (B) V12-Ras-NIH3T3 cells were incubated 50 µM PD98059 or 20 µM U0126 for 30 min and then cotransfected with mdr1b-Luc and pRL-Luc. Four hours after transfection the medium was replaced with fresh medium in the presence or absence of 250 nM doxorubicin (DOX), and 24 h later, the luciferase activities were determined as described in Fig. 1A. (C) V12-Ras-NIH3T3 cells were pretreated with DMSO or p38 inhibitor 20 µM SB2303580, or transiently transfection with a combination of JNK1AS and JNK2AS (JNK1+2AS) or control oligonuleotides (control JNK). The cells were then cotransfected with mdr1b-Luc and pRL-Luc, and the luciferase activities were determined as described in Fig. 1A. Each point is the average of multiple independent experiments; the error bars represent ± standard deviation. *p < 0.05 vs. V12-Ras, **p < 0.01 vs. vector, and ##p < 0.001 vs. V12-Ras.
|
The effect of mdr1b expression on cellular response to doxorubicin
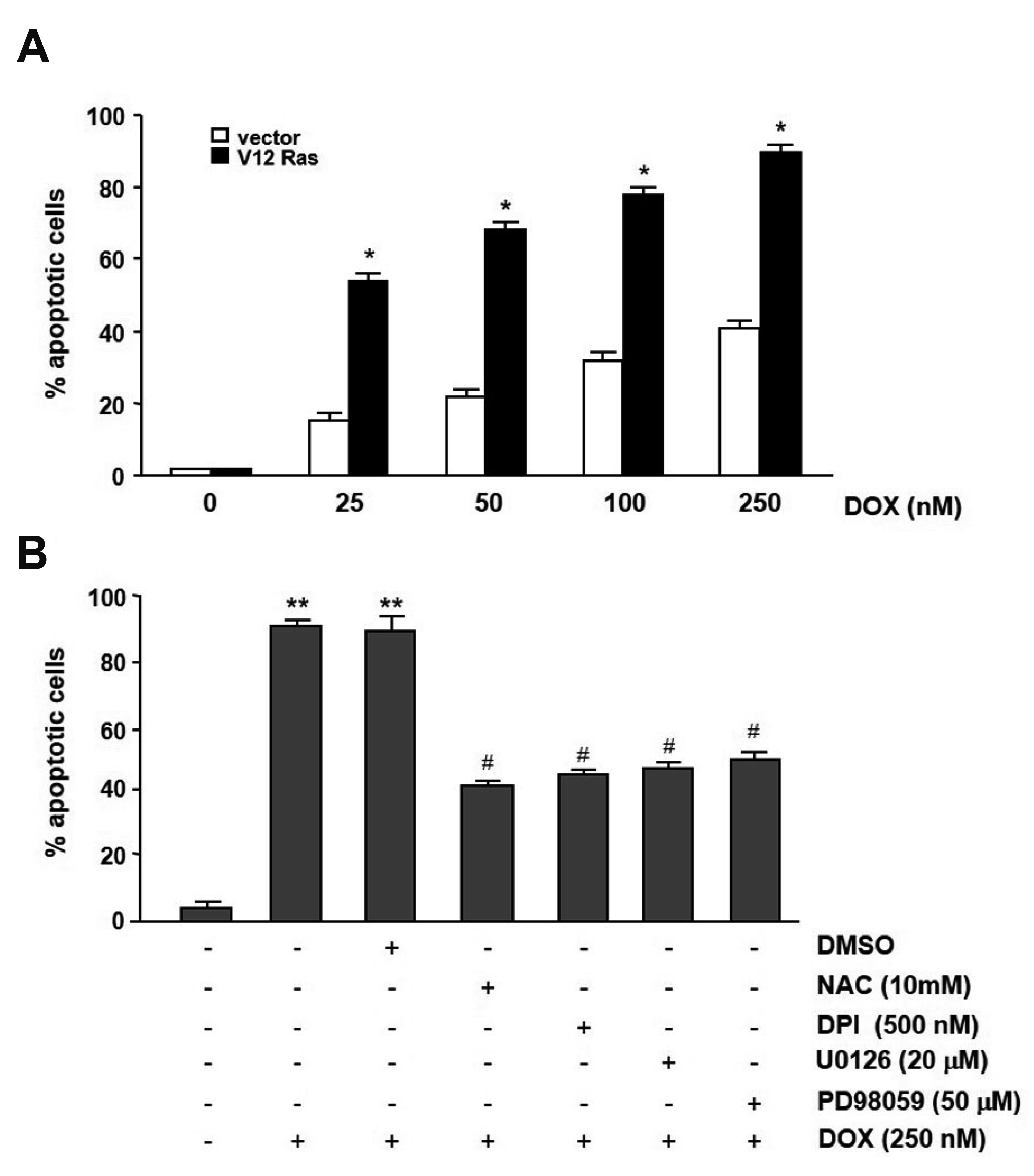 | Fig. 7Effect of mdr1b expression by doxorubicin on apoptotic cell death.(A) pcDNA3-NIH3T3 (vector) and V12-Ras-NIH3T3 (V12-Ras) cells were treated with the indicated doxorubicin (DOX) doses for 24 h. Subsequently, the cells were stained with propidium iodide, after which apoptosis was analyzed by flow cytometry. (B) Pretreatment of V12-Ras-NIH3T3 cells with 20 mM NAC, 500 nM DPI, 50 µM PD9850 or 20 µM U0126, and the apoptosis was then measured. The Bars represented standard deviation. values determined from at least three independent experiments. *p < 0.05 vs. vector, **p < 0.01 vs. DMSO, and #p < 0.005 vs. DOX.
|




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download